A new therapeutic strategy for Neuroblastoma
A malignant prognosis for people affected by Neuroblastoma due to the silencing of a specific protein (p53). Two innovative methods are used to restore p53 function with interesting results.
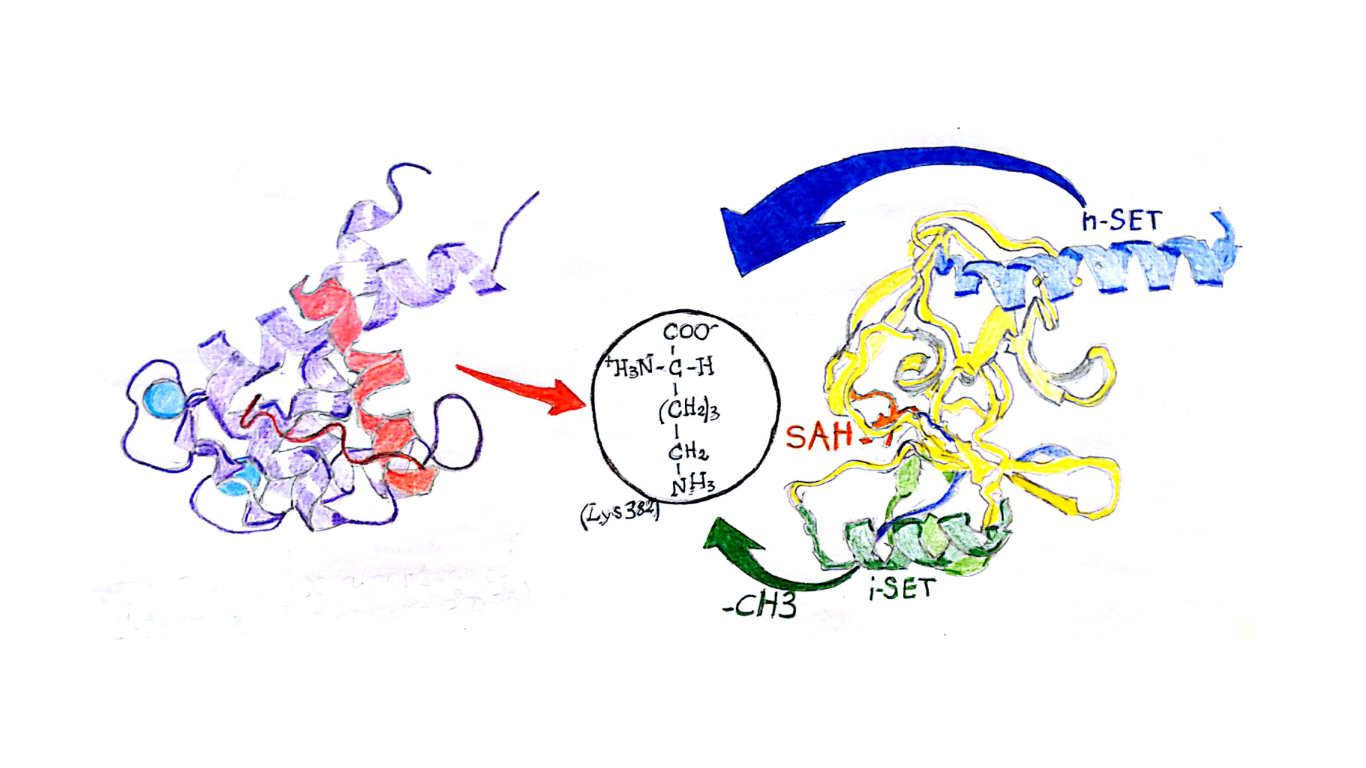
Neuroblastoma (NB) is a neural crest-derived tumor. The undifferentiated cells develop from one of the three embryo sheets, the ectoderm. Neural crest-derived cells are fast generator of the autonomic nervous system and others organs. For this reason, NB is a very aggressive tumor and its onset usually occurs in early life. Its incidence is about 10.2 cases per million children under 15 years old. Since 1980s, researchers thought that two genes were responsible for high risk of NB (HR-NB): ALK (Anaplastic Lymphoma Kinase), involved in the development of the brain and PHOX2B (paired like homeobox 2b), bindweed in the differentiation of neurons in embryo life. Mutation in both of them were associated with worst prognosis [1]. Nowadays, scientists focus their attention to the role of the protein p53. Such protein was identified in 1979 and it is also known as TP53 (tumor protein), or as “guardian of genome”. P53 is involved in the control of the genome stability during the G1 phase of the cell cycle. Its activity is regulated by MDM2, among others, that produces a negative control of the protein and inhibits its function. Another controller of p53 is p14ARF, that, conversely, activates p53 [2]. In Neuroblastoma disease, there are a lot of factors that generate the inhibition of p53 however the mechanism is not fully understood. In their work published in Cancer Cell last January, Veschi and collaborators [3] explored means to treat NB. They initially focused on MYCN (proto-oncogene) amplification, and mutations of TP53 gene. Above all, not all the subjects (HR-NB) have shown the amplification of MYCN, and also the mutation of TP53 gene occurred in less than the 2% cases. These observations brought their attention to another NB target: SETD8. This is a methyltransferase involved in many cellular and systemic processes [4]. In particular, methylation of histone H4, regulates gene transcriptions and DNA repair mechanism. SETD8 methylates p53 on the 382 Lysine in the C-regulatory domain. It is able to recognize the targets by the presence of the n-SET region in the SET domain. In SET domain there are other regions essential for the catalysis: i-SET and c-SET [5].
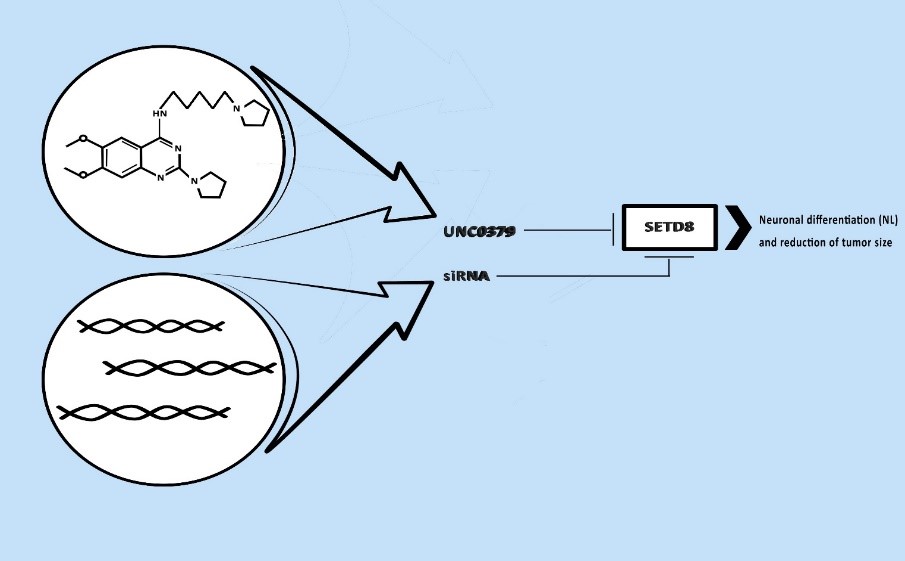
SETD8 is able to perform only a monomethylation, thanks to the formation of a water channel that joins the cofactor, SAM (S-AdenosylMethionine), and the lysine [6]. This prevents the capability of p53 to induce apoptosis in malignant cell transformation and promote NB. In their work, the authors tried to use a new epigenetic and pharmacologic approach to silencing SETD8 for an innovative treatment of HR-NB patients. The results obtained are the first step for change the ineffective current therapeutic approaches. In the first step the authors carried out a gene silencing with siRNA, (small double-stranded RNAs whose targets are mRNAs), in two different cell populations: SY5Y (MYCN-WT) and BE2C (MYCN-amplified). The results show that among 52 genes silenced, 16 reduce nuclei number (NN) and increase neurite length (NL). Surprisingly, SETD8 silencing exhibits the most effectiveness capacity of reduce tumor size and promote neuronal differentiation.
Furthermore, authors investigated the molecular pathways, after SETD8 silencing, with IPA (Ingenuity Pathways Analysis), a statistical tool, and GSEA (Enrichment Gene Set Analysis), that correlates a given set of gene in two biological states; observing as expected p53 pathways was enhanced. An evidence of rescued cell functions was observed with Western-blot (WB) analysis, a protein separation technique, showing a significant activity of caspases, that induce apoptosis. Next they used a pharmacological approach to inhibit SETD8 by UNC0379. This molecule was isolated in 2014 by a research group in North Carolina (Chapel Hill University) and in Toronto University [7]. UNC0379 is one of two selective inhibitors of the enzyme together with the first discovered nahuoic acid A. The difference is that UNC0379 is a competitor for the protein substrate of SETD8, while nahuoic acid A is a competitor for the cofactor SAM, thus effecting activity of many other methyltransferases.
In the first step the authors carried out a gene silencing with siRNA, (small double-stranded RNAs whose targets are mRNAs), in two different cell populations: SY5Y (MYCN-WT) and BE2C (MYCN-amplified). The results show that among 52 genes silenced, 16 reduce nuclei number (NN) and increase neurite length (NL). Surprisingly, SETD8 silencing exhibits the most effectiveness capacity of reduce tumor size and promote neuronal differentiation. Furthermore, authors investigated the molecular pathways, after SETD8 silencing, with IPA (Ingenuity Pathways Analysis), a statistical tool, and GSEA (Enrichment Gene Set Analysis), that correlates a given set of gene in two biological states; observing as expected p53 pathways was enhanced. An evidence of rescued cell functions was observed with Western-blot (WB) analysis, a protein separation technique, showing a significant activity of caspases, that induce apoptosis. Next they used a pharmacological approach to inhibit SETD8 by UNC0379. This molecule was isolated in 2014 by a research group in North Carolina (Chapel Hill University) and in Toronto University [7]. UNC0379 is one of two selective inhibitors of the enzyme together with the first discovered nahuoic acid A. The difference is that UNC0379 is a competitor for the protein substrate of SETD8, while nahuoic acid A is a competitor for the cofactor SAM, thus effecting activity of many other methyltransferases.
From an initial chemical screen, it appears that UNC0379 has a IC50 (Half Maximal Inhibitory Concentration) of 2µM, it means that it is powerful even in low concentration. SY5Y cells were treated with UNC0379 and the WB analysis shows increased p53 levels and decrease expression of SETD8, moreover an increase in neuronal differentiation and caspases activity. In this regard, UNC0379 showed a very similar effect to siRNA. However, the authors don’t explain how SETD8 is catabolised or how it is made ineffective. IPA analysis displays rescued p53 pathway activity. Interestingly, they also observed a different expression in the mevalonate and cholesterol pathways indicating unspecific action of UNC0379.
NB cells were treated with UNC0379, 2µM, then they were implanted in nude mice, to test the in vivo survival. Kaplan-Meier curves are used to indicate the survival of a population after a treatment and in this case they proved the therapeutic relevance of the inhibitor versus the control. The authors also inquired the possibility of synthetize siRNAs on command with a Tet-On system. It consists of a gene construct in which a transcription factor is able to bind the DNA, thus permitting the target gene expression, if it is bind by tetracycline or doxycycline. Mice fed with DOXY-chow showed long-lasting survival with respect to the mice fed with regular chow, as proven by Kaplan-Meier curves. Comparison between gene silencing and pharmacological inhibition demonstrates that gene silencing is more accurate and effective. In fact, 15 genes were differently regulated by siRNAs, instead of 10 by UNC0379, with 7 in common. Interestingly, some of these genes, with a well-known involvement in cancer onset, as JUN or BRCA1, were only down-regulated by siRNAs.
This paper shows an exciting discovery about a new possible drug, UNC0379, as a new molecule to reduce tumor size and induce cellular differentiation in NB. Nevertheless, there are important uncertainties about its lasting in vivo, its toxicity and its off-target such as mevalonate and cholesterol pathways. The authors pointed out that scarce bioavailability and uncertain animal toxicology in mice hindered direct in vivo treatment, that was not performed. For these reasons this study is preliminary although it was deepen-developed. Further studies are also required to implement the gene construct to express the siRNA in human therapeutic approaches or to modify UNC0379 structure in order to enhance its bioavailability and set up a pharmaceutical product.
References
[1] Maris J. M. et al., Recent Advances in Neuroblastoma, The New England Journal of Medicine, 2010.
[2] Okorokov A. L. and Orlova. E. , Structural biology of the p53 tumour suppressor, Elsevier, Current Opinion in Structural Biology, 2009.
[3] Veschi V. et al., Epigenetic siRNA and Chemical Screens Identify SETD8 Inhibition as a Therapeutic Strategy for p53 Activation in High-Risk Neuroblastoma, Cancer Cell 31, 2017.
[4] Van Maerkenmet T. et al., Escape from p53-mediated tumor surveillance in neuroblastoma: switching off the p14ARF-MDM2-p53 axis, Nature npg, 2009.
[5] Milite C. et al., The emerging role of lysine methyltransferase SETD8 in human diseases, Clinical Epigenetics 8:102, 2016.
[6] Girish T. S. et al., Multivalent Interactions by the Set8 Histone Methyltransferase with Its Nucleosome Substrate, Elsevier, Journal of Molecular Biology 2016.
[7] Zhang X. and Bruice T. C., Product Specificity and Mechanism of Protein Lysine Methyltransferases: Insights from the Histone Lysine Methyltransferase SET8, Biochemistry 47, 2008.
[8] Ma A. et al., Discovery of a Selective, Substrate-Competitive Inhibitor of the Lysine Methyltransferase SETD8, Journal of Medical Chemistry 57, 2014.
