A new ternary molecular complex in Aortic thoracic aneurism cells dysfunction
For the first time has been discovered the new HDAC9-BRG1-MALAT1 complex responsible for the smooth muscles cell dysfunction in Aortic thoracic aneurism, which would be a new target for epigenetic therapy of vascular diseases.
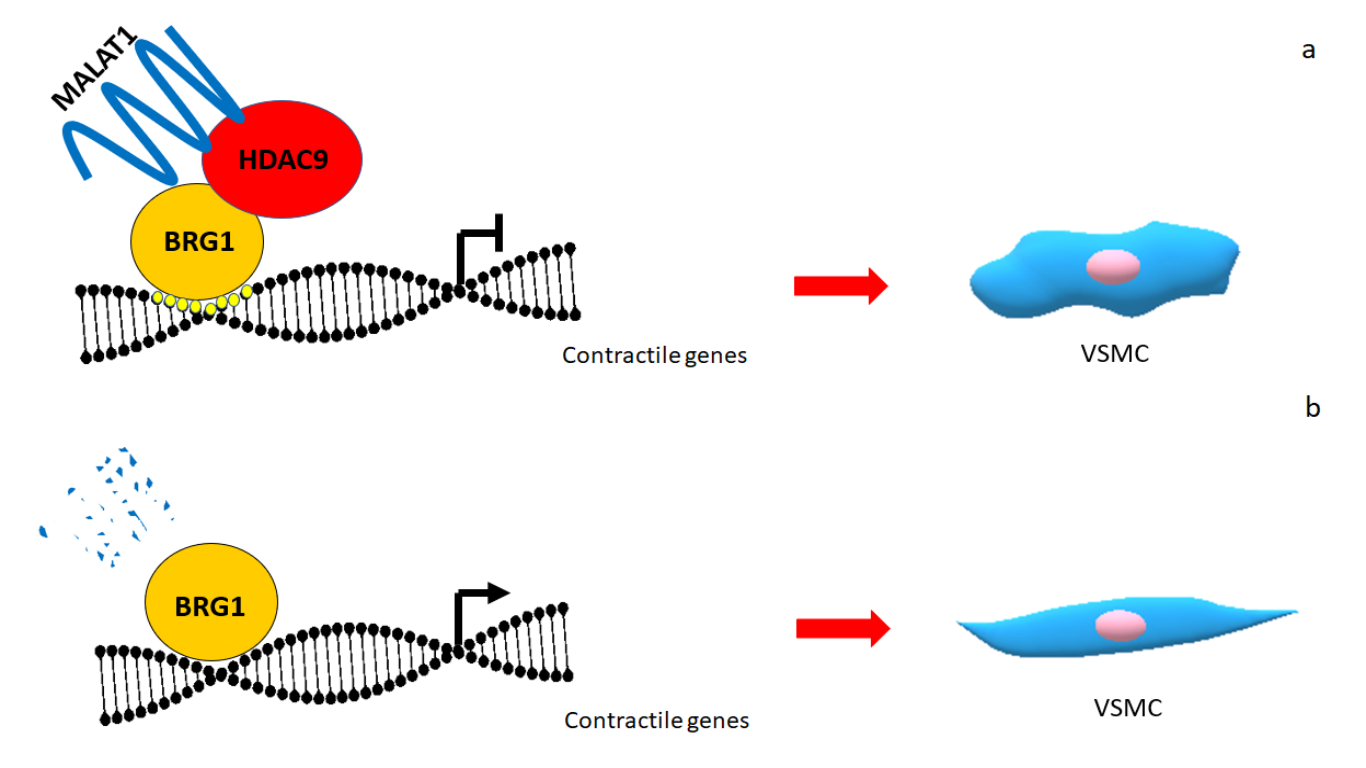
It remains obscure how the complex assemble and how it really works. To our knowledge, BRG1 is the element of the complex that binds to the nucleic acids on high conserved sequences [6]. HDAC9, that interacts with BRG1, is then the “recruiter” of PRC2 protein complex witch plays a role in epigenetic modifications, while MALAT1 with its triple helical motif at his 3’ end binds EZH2[7], the catalytic subunit of the PCR2 complex. This all bring to the trimethylation of the Lysine 27 on the H3 histone, epigenetic event well known to silence gene expression. It appears intriguing how this works but there is not a clear view on how all of this takes place, nor how to set in a temporal line all these events. We just know that MALAT1 is able to bind both BRG1 and HDAC9 and that it is the crucial link to the complex formation. It is easy to think MALAT1 as a target for therapy. However, besides the well known role of MALAT1 in cancer little is known about its role in aneurism. It will be useful to study in detail other forms of aneurism and see whether it forms the same or similar complexes to start thinking it as a new target for genetic therapy of aneurism. Until that we should focus our attention on HDAC9. It should be easier to ideate a new drug to interfere with the protein shuttling from the cytoplasm to the nucleus, sequestering HDAC9 in the cytoplasm reducing its accumulation in the nucleus to inhibit the complex formation
References
- Christian L. Lino Cardenas et al. An HDAC9-BRG1-MALAT1 complex mediates smooth muscle dysfunction in thoracic aortic aneurysm. Nature communications, 2018.
- Hang, C. T. et al. Chromatin regulation by Brg1 underlies heart muscle development and disease. Nature 466, 62 –67.
- International Stroke Genetics, C. et al. Genome-wide association study identifies a variant in HDAC9 associated with large vessel ischemic stroke. Nat. Genet. 44, 328–333.
- Consortium, C. A. D. et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat. Genet. 45, 25 –33.
- Foroud, T. et al. Genome-wide association study of intracranial aneurysm identifies a new association on chromosome 7. Stroke 45, 3194–3199.
- Filarsky, M. et al. The extended AT-hook is a novel RNA binding motif. RNA Biol. 12, 864–876.
- Wang, D. et al. LncRNA MALAT1 enhances oncogenic activities of EZH2 in castration-resistant prostate cancer. Oncotarget 6, 41045–41055.
