Anti-cancer therapy: resistance to DNA damage in renewable tissues
At the base of resistance to DNA damage in renewable tissues there is an increment of EZH2 levels, whose turnover is controlled by p53 and MDM2 proteins. In this way, are uncovered the epigenetic mechanisms responsible of cellular sensitivity [1]. In the fight against cancer, radio- and chemotherapy are certainly the most commonly used methods. Despite their success, they could be harmful to healthy cells and, in particular, to renewable tissues that are the most sensitive to DNA damage [2]. The purpose of the Kuser-Abali and colleagues’ work [1] has been designed to unveil an epigenetic mechanism to enhance healthy cells resistance to radiation and antitumoral drug-induced DNA damage. They analyzed three fundamental cellular components: p53, EZH2 and the MDM2/MDMX complex. It’s already known how, in renewable tissues, p53 levels must be downregulated to ensure the cellular proliferation because p53 acts as pro-apoptotic protein and oncosoppressor. For this reason, p53 levels are kept in a range that allows proliferation without interfering with sensitivity to DNA damage. This regulation is mediated by the combined action of MDM2 (Mouse double minute homolog 2) and MDMX (Mouse double minute homolog 4), that are the two chief regulators of p53 [3]. The other key component, EZH2 (Enhancer of Zeste homolog 2), is the catalytic subunit of PRC2 (Polycomb Repressive Complex 2) and it acts as histone-lysine N-methyltransferase enzyme that catalyzes the addition of methyl groups to histone H3 at lysine 27 (H3K27me3). The action of this complex promotes the formation of heterochromatin [4]: many studies demonstrated how modification of chromatin architecture is associated with development of DNA damage resistant phenotype [5].
Thus, p53 is a determinant of sensitivity to cellular damage and, in Kuser-Abali et al.’s study, is demonstrated how MDM2/MDMX-mediated regulation of p53 modulates this sensitivity by controlling EZH2 turnover. To this end, the authors used genetic and pharmacologic approaches to uncover the epigenetic mechanisms behind modulation of sensitivity to DNA damage. For the experiments are used a tamoxifen-inducible Cre knock-in mouse model mdmx C462. These mice show a single aminoacidic substitution on MDMX which prevents the formation of the complex with MDM2 maintaining, instead, the interaction with p53 [6, 7]. The prediction was that, after the exposure to ionizing radiation (IR) and to administration of doxorubicin in mdmx C462A/wt mice there was a greater p53 induction due to a higher tissue injury compared with the wild-type counterpart. In contrast, western blot analysis demonstrated a smaller increase of p53 in mutants related with a decrease of DNA damage and apoptosis, observable by using the nuclear marker γH2AX and TUNEL assay. Taken together, the data show how p53 responds in a quantitative manner to DNA damage [1].
It’s interesting to note how within these mutant mice there is an increment of chromatin condensation degree and how this effect is related to a higher DNA trimethylation (H3K27me3). Responsible of this histone modification is EZH2, highly expressed in bone marrow, spleen, thymus and duodenum. Observing how the resistance of these mice was related to the elevated methylation degree of the chromatin, the authors analyzed the regulator mechanisms behind EZH2 action. Because EZH2 is a protein involved in transcriptional repression, it must be dynamically regulated to permits the transcriptional activation in proliferative cells. The authors demonstrated that the EZH2 turnover requires the MDM2/MDMX complex formation that acts on it by ubiquitination.
The disruption of this complex by a small molecule (JBL5) leads to an increment of EZH2 levels and its half-life, determining in this way an increase of renewable tissues protection after the exposure to DNA-damaging agents. Because p53 levels are responsible for transcriptional activation of MDM2 [3, 8], mutations that cause the inactivation of p53 lead to a reduction of MDM2 levels and thus to an accumulation of EZH2, developing a resistant phenotype. The experiments demonstrated that EZH2 is one of the most important determinant in cellular sensitivity to DNA damage [1] and its pro-survival function is revealed in many cancer type [9, 10].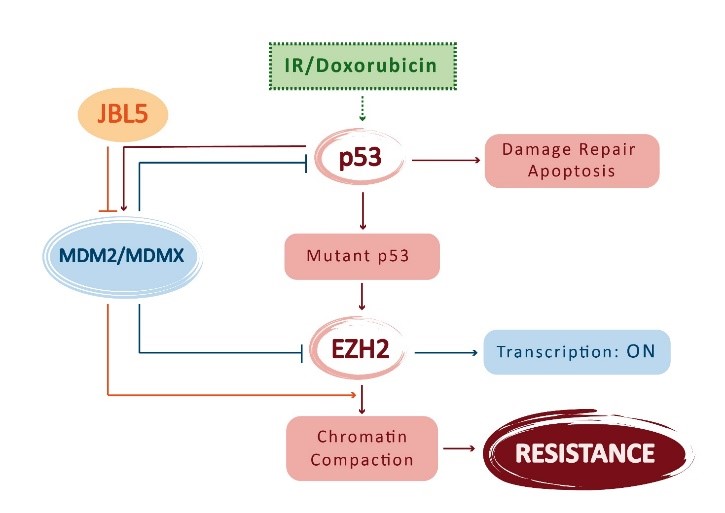
So, Kuser-Abali and colleagues’ study demonstrates how is possible to protect the healthy cells of renewable tissues from radio- and chemotherapy-induced DNA damage, opening new prospective about the using of drugs that can intervene on molecular mechanisms at the base of resistance. Is it possible apply all this results to patients? Is it possible administrate to patients, under anti-cancer therapy, drugs that alter in some way p53 and EZH2 regulation without changing the cellular equilibrium and decrease the treatment efficiency? Second, what could be the real cost of a genic and pharmacologic therapy like this? All these questions arise spontaneously because a specific clinical trial is not developed and used, yet. The more plausible future prospective previews the development and production of a drug designed on the base of JBL5 to permit EZH2 stabilization promoting tissues protection.
The pharmacologic approach could be the more efficient solution because to develop p53 mutations, and thus the genic therapy, is particularly complex.
References
- Kuser-Abali et al., “An EZH2-mediated epigenetic mechanism behind p53-dependent tissue sensitivity to DNA damage”, Medical Sciences (2018): http://www.pnas.org/content/115/13/3452 Proc Natl Acad Sci U S A.2018 Mar 27;115(13):3452-3457. doi:10.1073/pnas.1719532115. Epub 2018 Mar 14.
- Gudkov AV, Komarova EA, “The role of p53 in determining sensitivity to radiotherapy”. Nat Rev Cancer 3:117–129, (2003).
- Eischen CM, Lozano G, “The Mdm network and its regulation of p53 activities: A rheostat of cancer risk”. Hum Mutat 35:728–737, (2014).
- Ding X, et al., “The polycomb protein Ezh2 impacts on induced pluripotent stem cell generation”. Stem Cells Dev 23:931–940, (2014).
- Cann KL, Dellaire G, “Heterochromatin and the DNA damage response: The need to relax”. Biochem Cell Biol 89:45–60, (2011).
- Huang L, et al., “The p53 inhibitors MDM2/MDMX complex is required for control of p53 activity in vivo”. Proc Natl Acad Sci USA 108:12001–12006, (2011).
- Cao R, et al., “Role of histone H3 lysine 27 methylation in polycomb-group silencing”. Science 298:1039–1043, (2002).
- Kruiswijk F, Labuschagne CF, Vousden KH, “p53 in survival, death and metabolic health: A lifeguard with a licence to kill”. Nat Rev Mol Cell Biol 16:393–405, (2015).
- Lund K, Adams PD, Copland M, “EZH2 in normal and malignant hematopoiesis”. Leukemia 28:44–49, (2014).
- Bachmann IM, et al., “EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast”. J Clin Oncol 24:268–273, (2006).
