Bioenergetic regulation and neuronal differentiation NaBt mediated
An important phase of neurogenesis is neurite growth that requires a large quantity of energy. The authors induced this process through Nerve Growth Factor (NGF) treatment and evaluated NaBt effects. Results showed that the presence of many mitochondria, induced by NaBt treatment, promotes neurite outgrowing: this phenomenon was observed in cells subjected to a prolonged pre-treatment with NaBt that allows the acquisition of a useful mitochondrial number. So, the researchers also associated this behaviour with the timing of administration.
The CREB-Binding Protein (CBP), is a regulator of epigenetic state of chromatin through its acetyltransferase activity and in this study it was discovered that its expression is stimulated in NaBt-treated cells. It was also demonstrated that CBP is important to regulate number, morphology, and efficiency of mitochondria: in cbp-knockdown cells mitochondrial mass was lower and it was observable a swollen morphology associated to a defective activity of these organelles.
The last step was a bioinformatics study to analyse the pattern of the epigenetic mark H3K27ac on cells treated with NaBt for 3 days. The ChIP-seq analysis revealed a distribution of acetylated sites in different genomic regions: 13% introns, 18% exons, 25% promoters and 43% in intergenic regions. Subsequently, they performed a gene ontology analysis by PANTHER that revealed in particular an enrichment on cellular metabolic and neurotrophic genes. Further studies, based on STRING database, showed an enrichment on more neuronal genes and genes encoding mitochondrial ribosomal proteins (MRPs) involved on oxidative phosphorylation mechanism. Finally, researchers validated that NaBt promotes H3K27ac epigenetic mark, realizing a ChIP-qPCR analysis on 5 genes containing this modification.
In conclusion, this study has revealed an important correlation between epigenetic modifications, induced by NaBt, and activation of differentiation that involves both metabolic and bioenergetic processes in neuroprogenitor cells. This work represents an interesting starting point to elucidate the relationship between gene expression and metabolic processes on neuronal differentiation: it is an innovating and easy idea to act on metabolic, neurodegenerative and mitochondrial dysfunctions because it is based on the administration of a simple molecule like NaBt. However, remains a lot of work to do because there are plenty of unclear points. The results reported on this work are not sufficiently detailed and there are misunderstandings on image captions, so is difficult to draw exact conclusions.
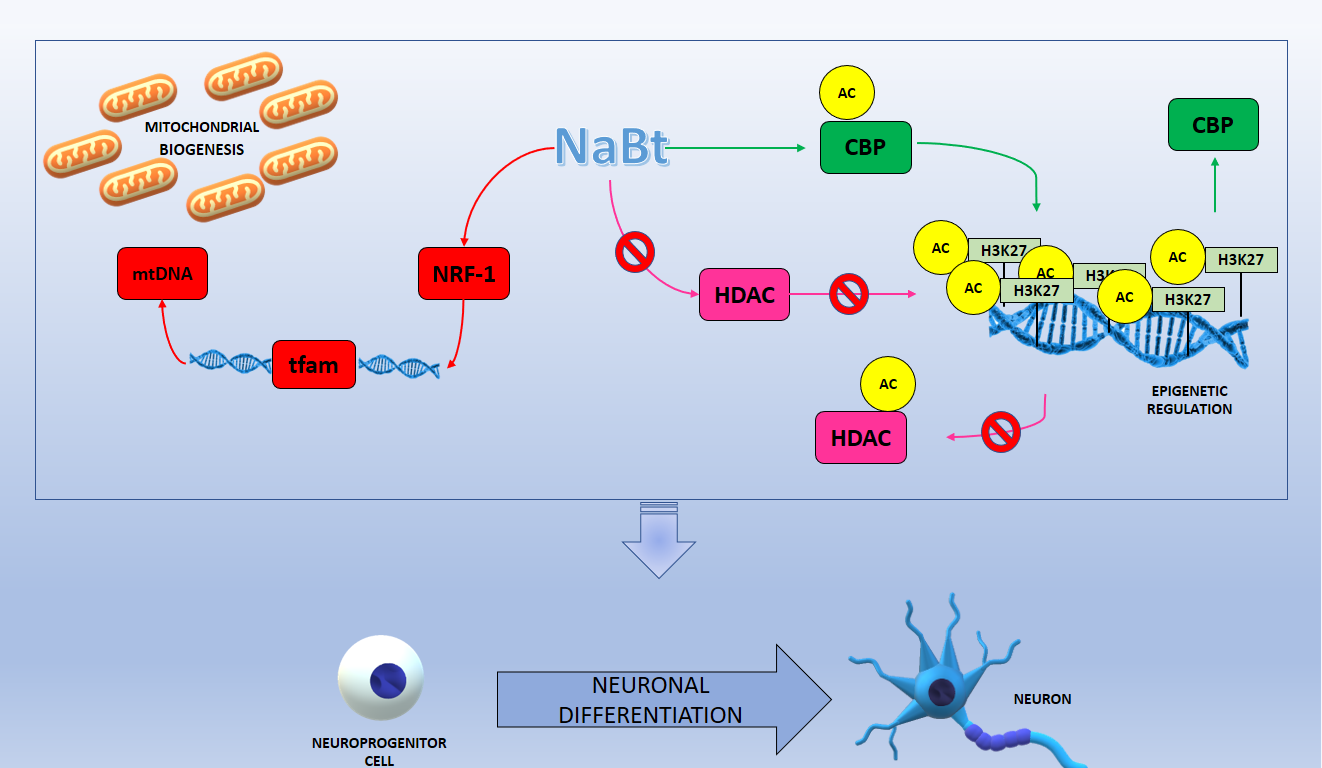
In this picture are summarized the processes induced by sodium butyrate, which in turn stimulate neuroprogenitor cells differentiation in neurons. In red the pathway that stimulates mitochondrial biogenesis is represented, while in green the one that controls DNA epigenetic regulation, in particular the acetylation on the histone H3 lysine residues (H3K27ac). Furthermore, the ability of NaBt to inhibit histone-deacetylases (HDAC) is shown in pink and this leads to an increase in DNA acetylation level
References
- Shen, J. Li and P. Casaccia-Bonnefil. Histone modifications affect timing of oligodentrocyte progenitor differentiation in the developing rat brain. J. Cell Biol. 169, 577-589 (2005).
- Larsson, J. Wang, H. Wilhelmsson, A. Oldfors, P. Rustin, M. Lewandoski, G. S. Barsh and D. A. Clayton. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nature genetics. 18, 231-236 (1998).
- K. Baxter, M. Uittenbogaard, J. Yoon and A. Chiaramello. The neurogenic basic helix-loop-helix transcription factor NeuroD6 concomitantly increases mitochondrial mass and regulates cytoskeletal organization in the early stages of neuronal differentiation. ASN Neuro. 1, 195-211 (2009).
- K. Baxter, M. Uittenbogaard and A. Chiaramello. The neurogenic basic helix-loop-helix transcription factor NeuroD6 enhanced mitochondrial biogenesis and bioenergetics to confer tolerance of neuronal PC12-NeuroD6 cells to the mitochondrial stressor rotenone. Exp Cell Res. 318(17), 2200-2214 (2012).
- M. Uittenbogaard, C. A. Bratner and A. Chiaramello. Epigenetic modifiers promote mitochondrial biogenesis and oxidative metabolism leading to enhanced differentiation of neuroprogenitor cells. Cell death and disease. 9, 360 (2018).
