Braf mutation and aging relationship in colon cancer
Fig. 1 – Malignant transformation of SSLs.
Abstract
Sessile Serrated Lesions (SSLs) are common colonic lesions present in patients of all ages. In their progression, these lesions acquire extensive DNA methylation alterations which can lead to the BRAF mutant colorectal cancer. Previous studies suggest that the age may influence the neoplastic transformation of SSLs, however, the underlying mechanisms are still unclear. In a recent publication, Fennel et al. (1) demonstrate that Braf mutation in a murine model accelerates the rate of DNA methylation drift favouring the progression of SSLs. The authors also assess that the age is more important than the length of time since the lesion is acquired.
Discussion
Sessile serrated lesions are premalignant lesions of the colon and they are commonly found by colonscopy in patients of all ages 2. From the SSLs, the BRAF-mutant colorectal cancers (CRCs) can arise, whereas the conventional adenomas usually present no mutations on BRAF. However, it’s still unclear if the malignant transformation of these lesions is more influenced by the length of time since the SSL initiation or by the age of patients. As the BRAF-mutant CRCs mostly occur in older individuals 3, the age is supposed to be the cause of SSLs progression.
A marked malignant potential and a CpGs island methylator phenotype, known as CIMP 4, are the two characteristics of an SSL. The extensive methylation induced by CIMP can silence key tumour suppressor genes, facilitating the malignant transformation of these lesions. Methylations can also alter the WNT signalling pathway, which is a putative target of epigenetic drift. The authors hypothesised that the silencing of tumour suppressor genes and WNT pathway dysregulation can dramatically increase the risk of malignant progression of SSLs.
In this study, the researchers used a murine model in which they have conditionally induced BrafV637E mutation to investigate how aging and DNA methylation alterations impact SSLs and their neoplastic transformation. At first, they analysed the methylome of their model using a reduced representation bisulfite sequencing (RRBS). They identified three types of methylation alterations:
- Type A methylation, that occurs in wild-type animals with aging.
- Type AB methylation, that is influenced by aging, but the rate of methylation changes increased with Braf mutation.
- Type B methylation, that is specific for Braf mutation.
Fennel et al. also performed enrichment analyses at pathway level using the PANTHER tool (Protein Analysis Through Evolutionary relationships) 5. They found that all three types of DNA methylation alterations involved in the WNT signalling pathway, which is crucial in the colorectal carcinogenesis 6.
As the authors found that Braf accelerated the DNA methylation drift that normally occurs during aging, they hypothesised that the small intestine of an elderly mouse would more rapidly develop murine serrated lesions (mSLs) following induction of Braf mutation rather than younger mice. Fennel et al. induced Braf mutation in animals either at wean (BrafW-5) or at 9 months (Braf9-14) for a period of 5 months. Next, they compared histology and DNA methylation of both groups and they found that younger mice rarely develop mSLs: the incidence of mSLs in BrafW-5 was 4.2%, whereas in Braf9-14 the incidence of mSLs was 43.8%, ten-fold higher.
Next, the researchers wanted to assess if the DNA methylation could increase the propensity of an aged small intestine to develop lesions. Intestinal tissue from a 10 months old mouse was harvested to produce organoids. The organoids showed an atypical cystic appearance; this phenotype may be due to the age of animals and to dysregulated Wnt signal 7. These organoids were treated with azacitidine, a DNA methyltransferase inhibitor and subsequently with 4-Hydroxytamoxifen (4-OHT) to induce recombination of the BrafV637E allele. After 72 hours, they observed that organoids treated with azacitidine and 4-OHT were significantly smaller.
After additional 11 days, in Braf mutant organoids (4-OHT alone) displayed high nuclear to cytoplasmic ratio, atypical nuclear appearance and multilayering of the epithelium. These results indicate that the inhibition of DNA methylation reduces the ability of Braf to induce proliferation probably by counteracting the epigenetic drift.
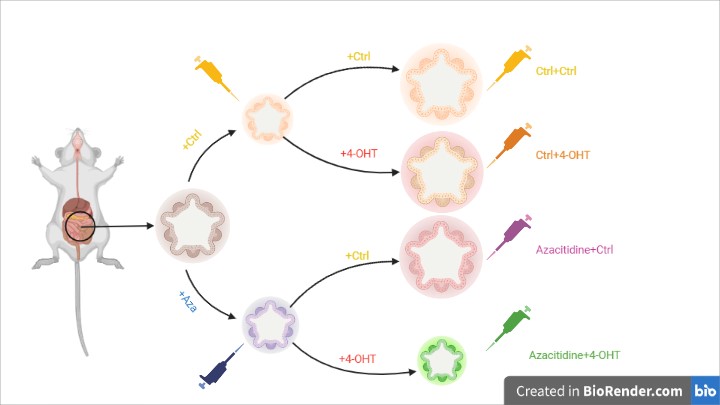
Fig. 2 – Inhibition of Braf mutant with azacitidine treatment.
Finally, Fennel and his group tried to examine the effects of BRAF mutation on epigenetic drift in human samples. They built an elastic net regression model, using three publicly available DNA methylation datasets, which allowed the authors to predict the epigenetic age. This model was applied to the DNA methylation data from SSLs, that showed an 11-years older epigenetic age with respect to the chronological age of the patients. Their hypothesis was that an advanced epigenetic age in SSLs due to BRAF mutation may increase the risk of malignant progression.
Conclusions
In conclusion, we underline three key points of this article:
- A putative target of epigenetic drift is the WNT signalling pathway; the epigenetic drift is accelerated by an exposure to mutant Braf.
- In a murine model with Braf mutation, an aged intestine had a higher incidence of developing a mSL if compared with younger animals.
- DNA demethylation via azacitidine can reduce the proliferation and dysplasia in organoids from aged mice.
Since the colorectal cancer is diffused especially in middle and late age individuals, it could be useful to investigate new preventive strategies for fighting this disease. A person predisposed to develop SSLs should receive a personalised and specific surveillance.
In the future, it could be necessary to explore new methods to inhibit the epigenetic drift, as Fennel et al. have done with azacitidine. These preventive programs can reduce cancer insurgence and aggressive chemotherapeutic treatments, that unfortunately are strongly invasive and debilitating for the patient.
References
- Fennell L., Kane A., Liu C., et al. Braf mutation induces rapid neoplastic transformation in the aged and aberrantly methylated intestinal epithelium. Gut 2021;0:1–14.
- Spring K.J., Zhao Z.Z., Karamatic R., et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology 2006;131:1400–7.
- Bettington M., Brown I., Rosty C., et al. Sessile serrated adenomas in young patients may have limited risk of malignant progression. J Clin Gastroenterol 2019;53:e113–6.
- Kambara T., Simms L.A., Whitehall V.L.J., et al. Braf mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut 2004;53:1137–44.
- Mi H., Muruganujan A., Ebert D., et al. Panther version 14: more genomes, a new Panther GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res 2019;47:D419–26.
- Borowsky J., Dumenil T., Bettington M., et al. The role of APC in Wnt pathway activation in serrated neoplasia. Mod Pathol 2018;31:495–504.
- Cui H., Tang D., Garside G.B., et al. Wnt signaling mediates the aging-induced differentiation impairment of intestinal stem cells. Stem Cell Rev Rep 2019;15:448–55.