Can human heart regenerate? A new possible strategy
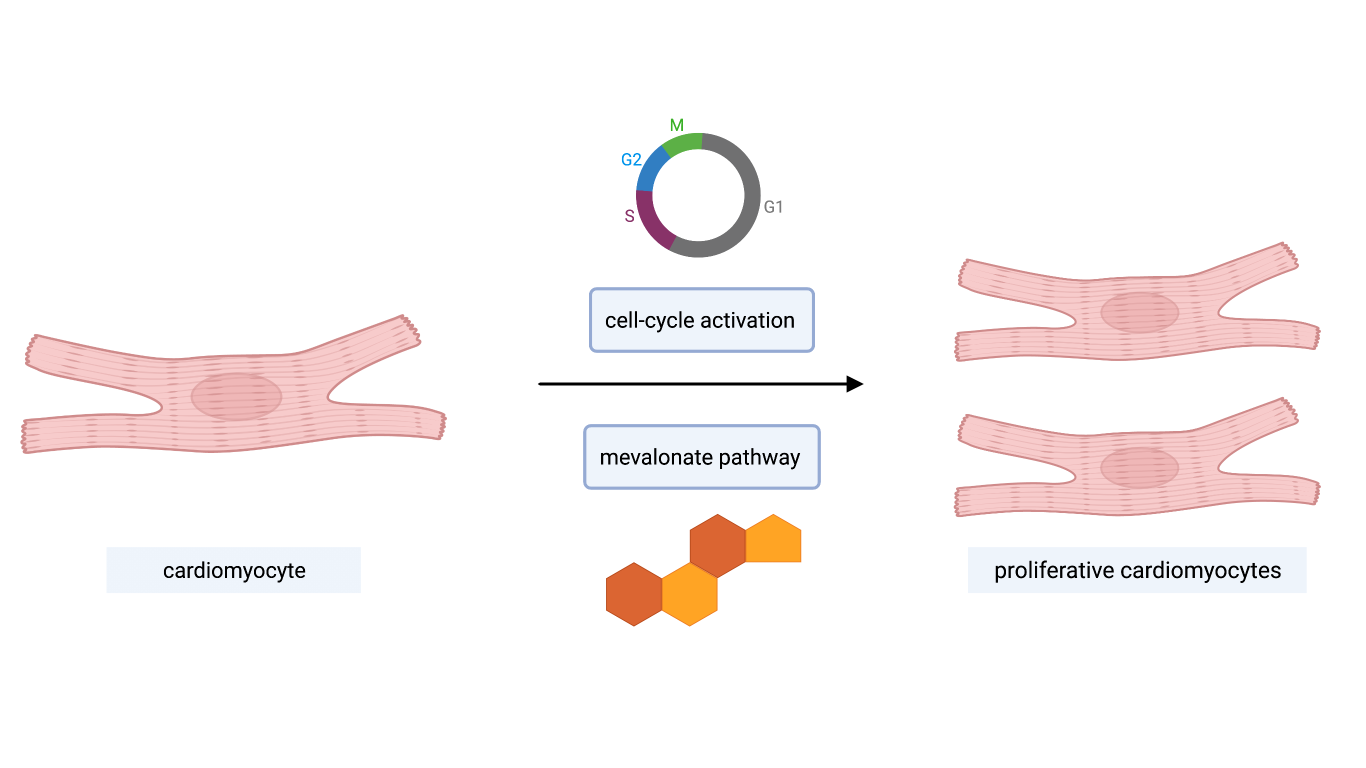
Figure 1 – Cardiomyocyte proliferation promoted by both a cell-cycle network and mevalonate (cholesterol) pathway activation
Abstract
Heart muscle does not regenerate following injury, which instead leads to heart failure, a principal cause of worldwide morbidity and mortality. The use of human cardiac organoids in drug screening studies can help researchers to choose a suitable pharmacological approach while investigating its biological and molecular role. In this study, Mills and collaborators identified two compounds activating both cell cycle and cholesterol biosynthesis pathway that are able to boost cardiomyocyte’s proliferation. This work opens new horizons in the identification of new therapeutic targets and new strategies for heart regeneration.
The World Health Organization reports that cardiovascular diseases (CVDs) are the number one cause of death globally, more than 30% of all deaths worldwide [1]. 85% of these cases arise out of heart failure. Lack of a successful pharmacological strategy worsens heart reluctance to regeneration. For this reason, there is a great interest in cardiac drug discovery. In 2019 the Australian research group headed by Hudson and Porrello published a scientific article in the Cell Stem Cell journal regarding their last research on this field [2].
In this study, Mills and collaborators employed 3D human cardiac cell cultures known as human cardiac organoids (hCO) to improve drug screening pipeline. This experimental system is more stable and effective than traditional 2D cell culture and allows a better comprehension of the molecular mechanisms acting in cells and tissues [3]. This is true especially for cardiovascular drug discovery and development [4]. Cardiac proliferation was assessed following different drug treatments. ~5000 compounds were initially screened in 2D human pluripotent stem cell-derived cardiomyocytes and then in immature proliferative hCOs, followed by validation in mature, fully differentiated hCOs. 105 small molecules were finally screened on hCO owing to their pro-regenerative potential. Noteworthy was the fact that some compounds, which induced proliferation in 2D, failed to induce it in the immature hCOs. If on one hand the pharmacological treatment triggers proliferation, on the other one this signal alters cardiomyocyte’s functionalities leading to an increased risk of arrhythmogenesis. As a consequence, the authors highlight the importance of screening compounds that promote proliferation without interfering with contractile parameters. From the list of 105 molecules, compound 3 and compound 65 were the two boosting proliferation in mature hCOs without adversely affecting contractile properties. In order to understand how these compounds drove the proliferation status, the authors performed RNA-sequencing (RNAseq) and single-organoid quantitative proteomics. Thanks to this analysis it was acknowledged that compound 3 regulated a mitogen-activated protein kinase 14 (MAPK 14) network, while upregulating the transcription of many cell-cycle controllers. Compound 65 regulated TGF-β and BMP networks, while inhibiting the transcription of the cell cycle inhibitor CDKN2B (Fig.2). Interestingly, compound 65 activated multiple enzymes in the mevalonate pathway (that is essential for the upstream of the cholesterol biosynthetic pathway) (Fig.2). The molecular profiles of the cells treated with the compounds 3 and 65 were compared to characterize the proliferation signature underlying the proliferation process. Integration of the data from cells treated with GSK3 and MST1 inhibitors, known to be cell cycle activators, expanded the molecular insights for compound 3 and 65. This analysis revealed a core overlap of 11 proteins, with 7 proteins belonging to cell-cycle networks, 2 proteins in the mevalonate / cholesterol pathway, and 2 nuclear transporters. This pharmacological approach indicates that both a cell-cycle and the mevalonate pathway have to be simultaneously active for cell-cycle induction in cardiomyocytes. This evidence underlined the role of the mevalonate pathway as a requirement in active cardiomyocyte proliferation.
Further proof of mevalonate implication came from another pharmacological assay. The research group proceeded by blocking the cholesterol biosynthesis pathway with simvastatin, a HMGCR inhibitor. Under these conditions, proliferation of cardiomyocytes was reduced as well as their size was affected. The presence of simvastatin also abolished the regenerative potential of compounds 3 and 65. Finally the experiment was brought from an in vitro to an in vivo system. Post-natal mice were injected with both compounds and an increase of DNA synthesis in the mouse cardiomyocytes was observed. The addition of simvastatin blocked the DNA synthesis confirming the importance of the mevalonate pathway. From a molecular point of view, additional experiments demonstrated how proliferation was dependent on YAP1, a known master regulator of cardiomyocyte proliferation.
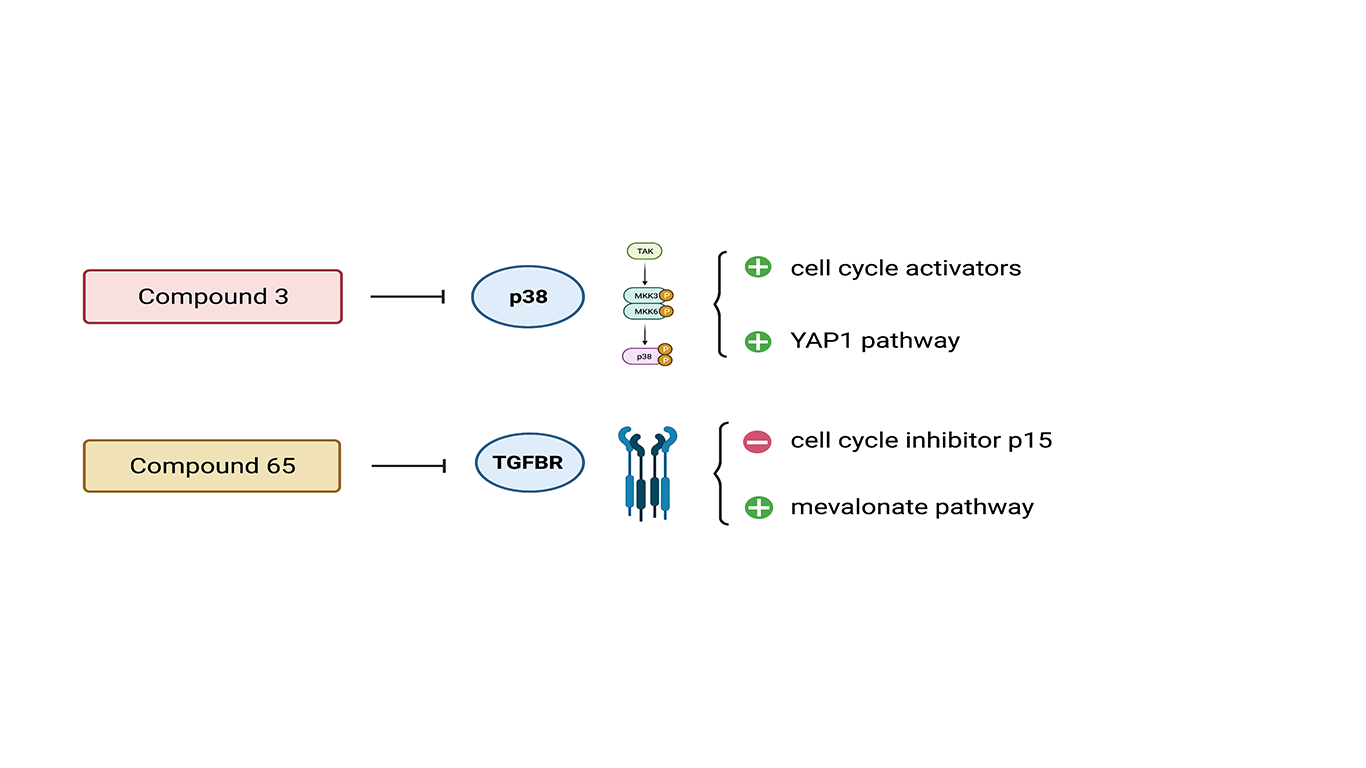
Figure 2 – Compound 3 acts as inhibitor of p38 signalling pathway leading to activation of cell cycle and YAP1 pathway (proliferation pathway). Compound 65 acts as inhibitor of TGFβR network which leads to a decrease of cell cycle inhibitor p15 and activation of mevalonate/cholesterol biosynthetic pathway. Both compounds are able to promote proliferation in vitro and in vivo.
In conclusion, this study is a further evidence of the importance of pluripotent stem cell-derived hCOs, an effective and predictive model for cardiac drug discovery. It allowed to identify several pro-proliferative compounds whose action triggered proliferation without impacting on force and contractility. Moreover, this model predicted that cell-cycle recovery in cardiomyocytes relies not only on the activation of a cell-cycle network but also on a functional mevalonate-cholesterol pathway (Fig.1). It suggested the active role of cellular metabolism as a triggering factor of the proliferation event. However, many aspects need further explanation. Even if compounds 3, 65 and other proliferative inducers were efficient in both in vitro and in vivo models, pharmacokinetics data do not sustain their possible usage as efficient and safe drugs. Different studies have explained how different miRNAs play several roles in stimulating cardiac regeneration [5]. It would be interesting to investigate the involvement of these molecules in stimulating the proliferation in cardiomyocytes. Finally, proliferative molecular mechanisms and other pharmacological strategies should be further investigated on different in vivo models such as infarcted or adult cardiomyocytes.
References
- World Health Organization, https://www.who.int. Accessed April 28, 2021.
- Mills RJ, Parker BL, Quaife-Ryan GA, Voges HK, Needham EJ, Bornot A, Ding M, Andersson H, Polla M, Elliott DA, Drowley L, Clausen M, Plowright AT, Barrett IP, Wang QD, James DE, Porrello ER, Hudson JE. Drug Screening in Human PSC-Cardiac Organoids Identifies Pro-proliferative Compounds Acting via the Mevalonate Pathway. Cell Stem Cell. 2019 Jun 6;24(6):895-907.e6. doi: 10.1016/j.stem.2019.03.009. Epub 2019 Mar 28. PMID: 30930147.
- Horvath P, Aulner N, Bickle M, Davies AM, Nery ED, Ebner D, Montoya MC, Östling P, Pietiäinen V, Price LS, Shorte SL, Turcatti G, von Schantz C, Carragher NO. Screening out irrelevant cell-based models of disease. Nat Rev Drug Discov. 2016 Nov;15(11):751-769. doi: 10.1038/nrd.2016.175. Epub 2016 Sep 12. PMID: 27616293.
- Fordyce CB, Roe MT, Ahmad T, Libby P, Borer JS, Hiatt WR, Bristow MR, Packer M, Wasserman SM, Braunstein N, Pitt B, DeMets DL, Cooper-Arnold K, Armstrong PW, Berkowitz SD, Scott R, Prats J, Galis ZS, Stockbridge N, Peterson ED, Califf RM. Cardiovascular drug development: is it dead or just hibernating? J Am Coll Cardiol. 2015 Apr 21;65(15):1567-82. doi: 10.1016/j.jacc.2015.03.016. PMID: 25881939.
- Afify AY. A miRNA’s insight into the regenerating heart: a concise descriptive analysis. Heart Fail Rev. 2020 Nov;25(6):1047-1061. doi: 10.1007/s10741-019-09896-w. PMID: 31838633.
- Figures created with BioRender.com