Cellular acetyl-CoA metabolism is related to memory formation in the hippocampus
Abstract
ACSS2 is an enzyme required for acetylation of histones. This process is related to metabolism (that provides acetyl-CoA) and regulates also memory consolidation and expression of early genes. ACSS2 is also located in different parts of the cells before and after differentiation and its position inside the cell is related to its functions.
Acetylation of histones is a mechanism that is important for many different cellular processes. Acetyl-CoA is required for this mechanism: it is a molecule composed by an acetyl moiety linked to co-enzyme A1.
Acetyl-CoA is provided by two enzymes: ACSS2 and ACL. ACSS2 (acyl-CoA synthetase short-chain family, member 2) is an enzyme that employs acetate to produce acetyl-CoA, in an ATP-dependent process. ACL (ATP citrate lyase) converts citrate into oxaloacetate and acetyl-CoA.
Using immunofluorescence in CAD cells, Mews and collaborators observed that ACSS2 and ACL have a different location inside the cell2. ACSS2 is cytoplasmatic in undifferentiated CAD cells but becomes nuclear during differentiation. Conversely ACL is cytoplasmatic both in undifferentiated and differentiated CAD cells. ACSS2 has a key role during neuronal differentiation, while ACL has a broad non-specific function for gene expression.
Acetylation is important for memory consolidation. In fact, ACSS2 is expressed throughout the hippocampus and is the enzyme that supplies acetyl-CoA for the acetylation. The researchers demonstrated that histone acetylation can be controlled by changing levels of nuclear acetyl-CoA; ACSS2 binds to chromatin and provides the molecule for local HAT activity. The enzyme recycles CoA and recaptures acetate from deacetylation reactions.
To investigate histone acetylation during differentiation of CAD cells into neurons, and the direct association of ACSS2 to chromatin, they performed a ChIP-seq protocol for histones H3K9ac, H4K5ac, and H412ac. Two ACSS2 antibodies showed a high correlation overlapping peaks for MACS algorithm.
Binding of ACSS2 with histones correlated with increases in acetylation in differentiated relative to undifferentiated CAD cells, for instance at the promoters of Nudt1 and Tab2 genes, both linked to neurodegenerative diseases3. Gene ontology analysis showed that genes proximal to ACSS2 peaks were linked to neuronal differentiation, and that ACSS2 may provide acetyl-CoA to HAT enzymes.
80% of ACSS2 peaks upstream of the nearest target gene and downstream the transcription start site overlapped an acetylation peak2.
In addition, the ACSS2 peaks that directly overlapped H3 and H4 acetylation, suggests that H4 acetylation might be most responsive to ACSS2-derived acetyl-CoA.
It was also performed ChIP-seq over the Camk2a locus, which encodes the CaMKII α-chain required for hippocampal long-term potentiation, showing an acetylation overlapping peaks in concomitance of ACSS2 only in the CAD differentiated cells. To confirm the results, it was used an ACSS2-inhibitor (ACSS2-i). Upon this treatment, the 299 genes that showed a reduced expression were those with the greatest differentiation-linked increases in histone acetylation. Furthermore, it was found a relationship between ACSS2 enrichment and increased gene expression in CAD differentiated cells using multiple linear regression analysis2.
The levels of nuclear acetyl-CoA in both ACSS2-knockdown cells and ACSS2-i cells were similarly decreased, supporting the theory that ACSS2 enzymatic activity supplies nuclear acetyl-CoA. ACSS2 was co-immunoprecipitated with acetylated chromatin, and CBP (CREB binding protein) showing a physical association in differentiated CAD cells, this suggests that ACSS2 binds chromatin at transcriptionally active genes to increase memory formation in vivo4,5.
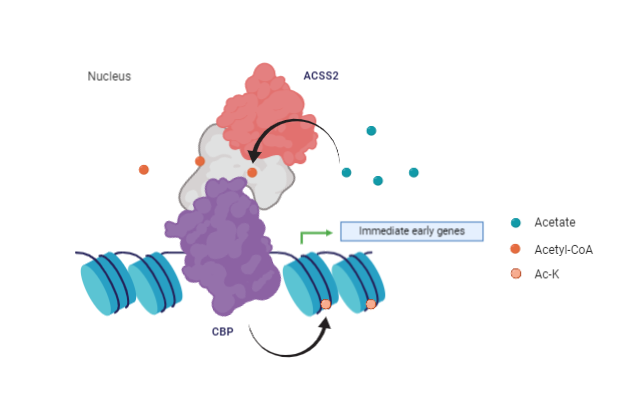
ACSS2 is related to object location memory and upregulation of immediate early genes in the dorsal hippocampus.
Hippocampal memory consolidation also requires the transcription factor CREB and the coactivator CBP (CREB binding protein) for acetylation activity and this mechanism can upregulate immediate early genes. Decreasing levels of ACSS2 in dorsal hippocampus, mice did not show many behavioral alterations. On the contrary, analyzing spatial memory with an object-location experiment, knockdown mice showed a reduced novelty associated with object’s shift to another position2.
In conclusion, the author showed that metabolic state can regulate chromatin structure inducing histone modifications, suggesting a connection between cellular metabolism and neuronal plasticity. ACSS2 functions as a chromatin-bound transcriptional coactivator by acetylating histones and has a role in upregulating immediate early genes connected to neuronal plasticity and memory consolidation.
References
- Pietrocola, F., Galluzzi, L., Bravo-San Pedro, J. M., Madeo, F. & Kroemer, G. Acetyl coenzyme A: a central metabolite and second messenger. Cell Metab. 21, 805–821 (2015).
- Philipp Mews, Greg Donahue, Adam M. Drake, Vincent Luczak, Ted Abel & Shelley L. Berger Acetyl-CoA synthetase regulates histone acetylation and hippocampal memory Nature 546, 381-385 DOI:10.1038/nature22405 (2017).
- Sardi, S. P., Murtie, J., Koirala, S., Patten, B. A. & Corfas, G. Presenilin-dependent ErbB4 nuclear signaling regulates the timing of astrogenesis in the developing brain Cell 127, 185–197 (2006).
- Gräff, J. & Tsai, L.-H Histone acetylation: molecular mnemonics on the chromatin Rev. Neurosci. 14, 97–111 (2013)
- Vecsey, C. G. et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J. Neurosci. 27, 6128–6140 (2007).