Channelling the power of evolution for the synthesis of a new world
Using the “enzyme direct evolution” technique has been created a variant of cytochrome P450 capable to catalyze a regioselective alcylation on a C-H bond on a sp³ carbon using a diazo compound to deliver the alkyl group.
The group of F. Arnold directed by Ruije Zhang has tried successfully to create an enzyme able to catalyze the regioselective alkylation on a sp³ carbon attaching an etyl diazo acetate [1]. The starting point of the procedure was to find a protein that could naturally catalyze a regioselective reaction on the sp3 C-H bond. In past studies several procedures have been already attempted but none led to satisfactory results regarding the yields and costs of the processes [2,3]. This team tried a new approach, they decided to use a heme protein. This super-family, due to the heme group, is naturally capable to catalyze a regioselective bond formation on a sp³ C-H bond [4]. In presence of a diazo compound some of these proteins are also able to catalyze the transfer of the alkyl group of the diazo compound on a specific carbon of another substrate. The idea has been to modify the protein to improve the regioselectivity and the turnover number of the alkylation reaction to obtain an enzyme that could be used in the field of synthetic biology with possible applications both in the chemical and pharmaceutical industries [1].
To make it happen the group have screened a large number of heme proteins, to seek for the best catalyst for the reaction, belonging to three enzymatic superfamilies: Cyp 450, globin, and Cyp c families. This process as lead the researchers to a subfamily of cytochrome P450 called P411 found in the bacteria Bacillus megaterium in which the axial cysteine ligand is substituted by a serine [5]. Inside this cluster of P411 enzymes they have found a particular variant that by his natural disposition had a good turnover number and stereoselectivity to the alkylation reaction with a diazo group. This enzyme has been used as the starter for the “enzyme direct evolution” technique [6]. The first five rounds were unsuccessful but after the depletion of the FAD domain of the protein and eight mutations cycles the team has produced the P411-CHF variant (Fig. 1).
This new enzyme has a high stereoselectivity (94% average) for many substrates like aryl rings, alkyls and amine also maintaining a good TTN (tournover) which can change from 500 to more than 3000 depending on the substrate. The alkyls substrates had the higher TTN due to the higher flexibility of their molecular structure that helped in the access of the catalytic site; while the higher stereoselectivity has been fuond using the aryl rings as substrates. In this pioneering work the reserchers have demonstrate that is possible to use the evolutionary proccess to push the reactivity of an enzyme in the direction desired achienving really high results in a moderate time. In the paper is not analyzed the reaction mechanism that allows the alkylation process. Further studies has been conducted by Giorgio Capaldi and Lorenzo Lamberti using Chimera and Yasara to deepen this process comparing the starting enzyme whith the P411-CHF. The result were controversial due to a mutation, S438T, located near the reaction site (figure 2).
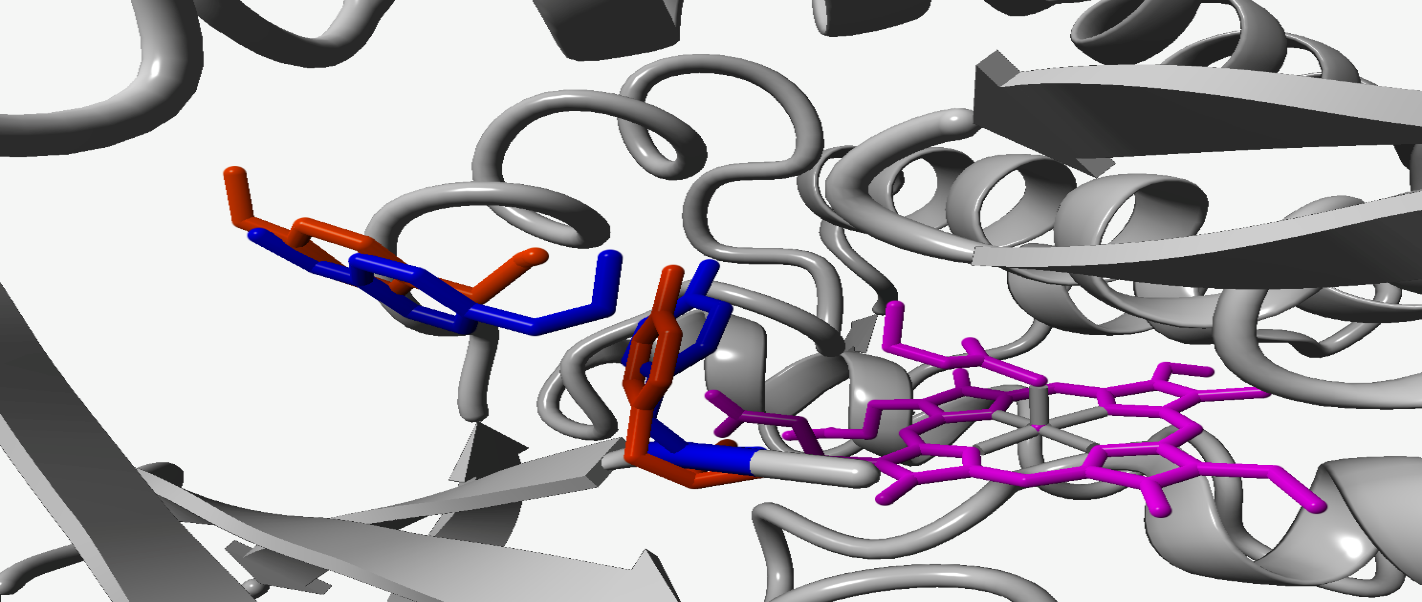
Fig. 1 mutated amino acid (S438T) in the catalytic center of the Cyp P411-CHF. The heme group is shown in pink, while the substrate and the phenolic ring of the mutated amminoacid are shown in red and in blue is shown the change of position after performing a computational operation to minimize the molecular energy.
This change blocked the way to the entry channel of the substrates in the active site because the tyrosine is more bulkier than the serine. Using a minizimation energy function we saw that the benzene ring of the tryptofane tends to rotate allowing the substrate to pass into the reactive site. This is just a theory and the article did not mention nothing about the impications of the mutations on the catlytic process. However if the group had tried to do some computational studies on this protein it could helped to undestand how the reaction is catalyzed and they could predict the behaviour of the enzyme on different substrates. Instead the group made many in vitro tests to analize the reactivity of the enzime with different substrates spending money and time that could have been saved by performing the computational tests first.
The applications of this particular enzyme are to be searched mostly in the pharmacological field, since the C-C bond is the base for every organic compound. In the paper it has been described a possible pathway for the synthesis of lyngbic acid and cusparine, both of these molecules have interesting properties and have been difficult to produce or extract with a suitable mechanism [1]. This enzyme can be used also in semisinthetic synthesis of antibiotics, werefore the possibility to attach new alkylic groups to an antibiotic without inhibing his activity could be the key for the resistence problem. This phenomenon is becoming one of the biggest challeges of the new millenium, the ability to create different forms of the same antibiotic with a high stereo-enantio selectivity can ensure the production of new semi-synthetic antibiotics that can affect also resistant strains to the canonical antibiotics [7].
Through the creation of a poly-enzyme complex it is possible to exploit the activity of the Cyp-411 CHF in a modification process of a substrate that includes numerous stages. This appraoch would speed up the entire catalytic process.
The possible uses of the ability to catalize the C-C bond starting from a C-H bond on a sp³ carbon are very wide; however the horizon of possibilities widens immensely if we evaluate all the different known enzymes and the different evolutionary paths in which I can drive them through the “enzyme direct evolution” technique.
References
- Ruijie K. Zhang, Kai Chen, Xiongyi Huang, Lena Wohlschlager, Hans Renata & Frances H. Arnold. Enzymatic assembly of carbon–carbon bonds via iron-catalysed sp3 C–H functionalization. Nature (2018)
- Hartwig, J. F. & Larsen, M. A. Undirected, homogeneous C–H bond functionalization: challenges and opportunities. ACS Cent. Sci. 2, 281–292 (2016).
- Liao, K. et al. Site-selective and stereoselective functionalization of non- activated tertiary C–H bonds. Nature 551, 609–613 (2017).
- Poulos, T. L. Heme enzyme structure and function. Rev. 114, 3919–3962 (2014).
- Coelho, P. S. et al. A serine-substituted P450 catalyzes highly efficient carbene transfer to olefins in vivo. Chem. Biol. 9, 485–487 (2013).
- Frances H. Arnold. Directed Evolution: Bringing New Chemistry to Life. (2017)
- Çig˘dem Yılmaz, Gülay Özcengiz: Antibiotics: Pharmacokinetics, toxicity, resistance and multidrug efflux pumps. Elsevier Biochemical Pharmacology 133; 43–62 (2017).