Disordered regions in transcription factors lead to short-order binding
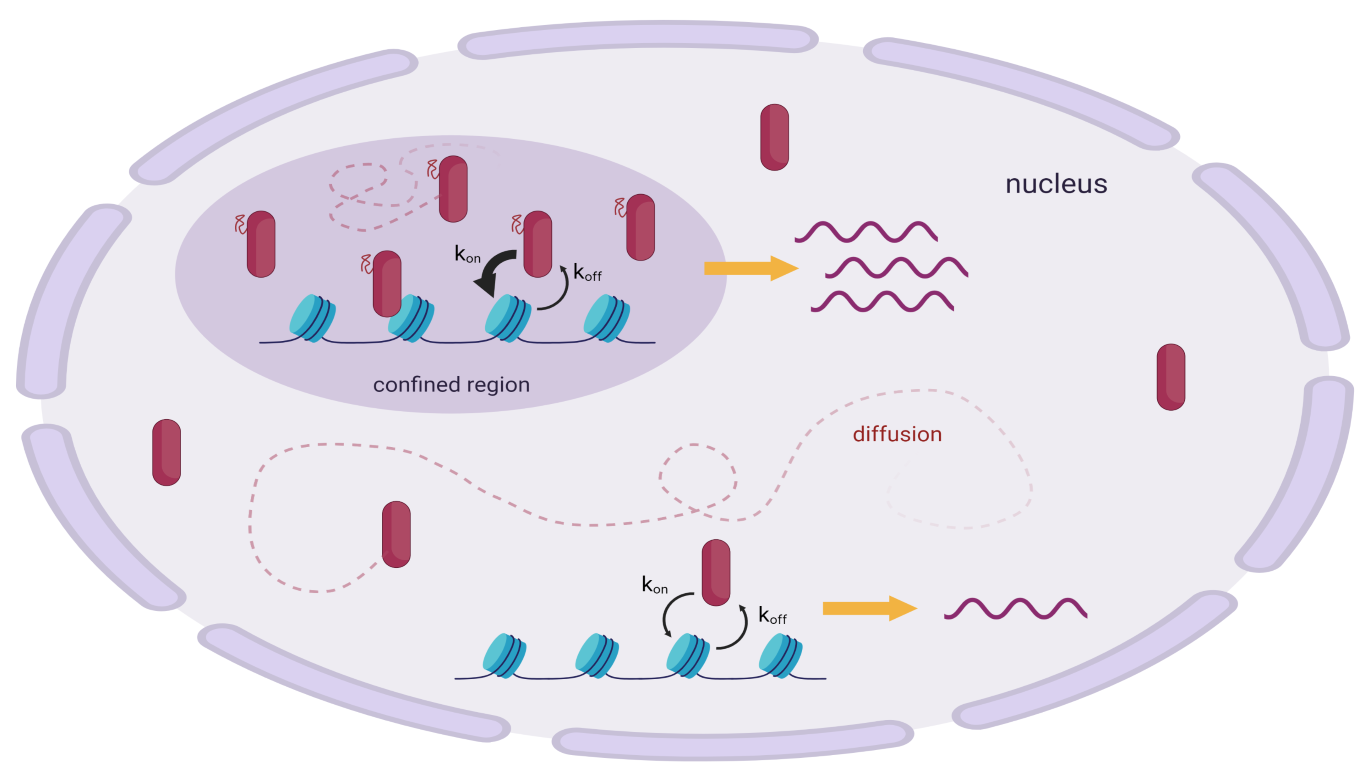
Figure 1 – Different behaviours of transcription factors into the nucleus. TFs can either diffuse or interact with chromatin and between each other, giving rise to compartments characterised by higher concentrations. (Created with BioRender.com)
Abstract
IDRs (intrinsically disordered regions) are flexible aminoacidic sequences that lack a well-defined rigid structure. Their presence in proteins has been demonstrated to be fundamental for many biological processes, including transcription regulation: indeed they are present in many different types of transcription factors. Combining single-molecule tracking with a machine-learning-based approach, Garcia et al. (from the Laboratory of Receptor Biology and Gene Expression at the National Cancer Institute) were able to analyse and characterise the dynamics of transcription factors into the nucleus, focusing especially on glucocorticoid receptors. This led to the discovery of four populations on the basis of the different motility of molecules. Unexpectedly, two of these populations showed a sub-diffusive behaviour: the slower one is due to chromatin binding, whereas the faster one is given by the interactions among IDRs.
Discussion
IDRs are fundamental for many interactions into the cells, as they can assume many different conformations due to the lack of a precise secondary structure. IDR-IDR interactions usually give rise to high-density aggregates (also called nuclear foci), which are membraneless micron-scale compartments organised through liquid-liquid phase separation, and kept together through different macromolecular interactions. These compartments are typical of eukaryotic cells and are probably implied in many different biological processes, including transcription [1].
Glucocorticoid receptors (GRs) are ligand regulated nuclear receptors, and they are inactive until they bind to a specific activator. In their inactive form they are monomers and are located in the cytoplasm; as an activator enters the cell and gets in contact with the receptor, a conformational change occurs, leading to the detachment of an inhibitor and to the dimerization of two receptors. After that the dimer is ready to enter the nucleus and activate transcription [2]. However, how they navigate into the nucleus “looking for” their consensus sites on chromatin still remains unclear. Moreover, given that GRs contain IDRs, it remains to clarify whether they play a role in the transcription processes regulated by GRs.
A useful tool to obtain insights on the movement of molecules into cellular compartments is single molecule tracking. Single molecule tracking (SMT) is a high-resolution technique that allows to visualise, thanks to specific probes such as fluorochromes, the movements of molecules into living cells. As the name says, this technique does not simply report an average value, but every single molecule gives a signal, which is tracked down. This technique represents a fundamental achievement for studying the movements of molecules into cellular compartments. For example previous studies on liquid-liquid phase separation [See the article – Is the analysis of liquid liquid phase separated chromatin useful to study in vitro the complex in vivo chromatin organization?] within the nucleus were performed through the photobleaching technique, which however reports only an average result and does not allow to clusterize the data obtained.
In a recent paper, Garcia et al. [3] exploited this technique to directly follow GRs moving into the nuclear environment. During their roaming into the nucleus GRs can either diffuse or also interact with other proteins, coregulators, RNA, chromatin and so on, and each behaviour is reflected by a different kinetic, which can be measured through SMT. Indeed, understanding how they move is the first step to discover how they interact.
The motility of molecules can be described through the mean squared displacement (MSD). When the MSD value is linearly proportional to time, the molecule simply diffuses, whereas if the relation between MSD and time is not linear, the movement can be either sub- or super-diffusive.
The data obtained from the SMT experiments were analysed by Garcia et al. [3] through an unsupervised machine learning algorithm called Perturbation expectation maximisation (pEM), which is specifically designed to clusterize data without needing to pre-determine the total number of clusters. This is particularly important to obtain unbiased results, because forcing the algorithm to detect a certain number of clusters can result in contrived results. Indeed Gracia et al. expected only two of the four populations, whereas the other two would not have been detected without leaving the cluster number as a free optimizable parameter.
From the experiment it came out that there exist two diffusive populations and two sub-diffusive populations: however, as the SMT microscopy only focuses on a single plane, the technique does not allow to follow molecules that move with fast kinetics. The higher the speed, the higher the probability that molecules will exit the focal plane resulting in loss of signal. For this reason only sub-diffusive molecules were further investigated.
The presence of one sub-diffusive state, due to chromatin binding, was not a surprise, but the second sub-diffusive behaviour was quite unexpected.
Garcia et al. [3] verified that the slowest sub-diffusive behaviour is due to chromatin binding by repeating the SMT experiment using a mutant unable to bind chromatin and exploiting the hormone withdrawal technique.
Assuming that GRs contain IDRs, and that IDRs are involved in the formation of aggregates, they hypothesised that GRs involved in aggregates could show a sort of confinement, and therefore exhibit a sub-diffusive behaviour. A possible way to test this hypothesis is repeating the SMT experiment with a mutant which does not contain its IDR, or with a transcription factor which naturally lacks an IDR in its sequence. Both the experiments confirmed the hypothesis that IDR-IDR interactions are responsible for the “faster” sub-diffusive behaviour.
The fact that the presence of IDRs in the structure of GRs leads to the formation of aggregates, in which the particles move at a lower speed, means that also other IDR-containing proteins could show the same behaviour. Another well-known class of IDR-containing proteins are histone proteins. SMT analysis revealed that actually they do show the same two sub-diffusive behaviours, and also that the confined population is highly represented, supporting the hypothesis that also histones are subjected to confination phenomena in addition to their incorporation into nucleosomes. Possibly, the presence of a confined population of histone proteins around chromatin could be a mechanism by which the cells are able to accelerate the process of re-assembling histones and nucleosomes after the passage of the polymerase on chromatin.
SMT experiments are useful not only to provide information about kinetics, but also for the calculation of dwell-time distribution (also called survival distribution), which represents the time that a protein stays in the bound state. So far, the major part of transcription factors have been described to exhibit a bi-exponential survival distribution. Garcia et al. [3] demonstrated that this model is appropriate to fit the dwell-time distribution of transcription factors that can only bind highly conserved motifs or generic chromatin in aspecific way. However, transcription factors that can also bind to more degenerated consensus motifs show instead a power-law dwell-time distribution. They also showed that mutants without IDRs can bind only very strong sequence motifs and show therefore a bi-exponential behaviour, whereas the presence of an IDR in the structure of the protein increases the heterogeneity of binding sites and results in a power-law model. A possible explanation is that maybe IDRs are involved in the recruitment of some extra transcription factors that can help the binding to chromatin.
Garcia et al. [3] also proposed that the chromatin-bound population and the GRs present in the nuclear foci could show a cooperative relation. On one hand, the GRs bound to their promoter may serve as nucleators leading to the formation of nuclear foci in the surrounding area. This is maybe possible because the receptor interacts with DNA with its C-terminal domain, whereas the N-terminal domain, i.e. the IDR, is free to interact with the IDRs of the non-bound population. On the other hand, the presence of a pool of non-bound GRs in the area around the promoters can increase the kon of binding, which is the rate at which receptors actually bind to chromatin, possibly leading to a higher transcription rate.
It is relevant to notice that nuclear foci only influence the kon, but neither the koff (rate of dissociation) nor the residence time (i.e. how long a protein stays in the bound state).
IDR-mediated confinement is probably a natural mechanism that GRs can use to regulate gene expression in a more efficient way. This is achievable modulating the concentration of proteins in the neighbouring region and restricting the exploration area. This mechanism was also proved for other transcription factors, such as CBX2 [4], and seems to be quite common in nature.
Conclusions
Some aspects still remain to be clarified. First of all, whether the IDR-IDR interactions are the only mechanism that regulates the formation of nuclear foci. Actually, these interactions may be not sufficient to determine the formation of these compartments, and other types of interactions can be involved. Moreover, some studies [5] have proved that the presence of IDRs is not even necessary for the formation of nuclear foci, although they seem to be very important for their stabilisation.
In addition, it could be interesting to further analyse the two diffusive populations, in order to understand why there are actually two, and also to determine whether there exist also some super-diffusive populations due to active motion.
Finally, one fascinating point might be the possibility to deduce the residence time from the SMT experiments. Garcia et al. [3] demonstrated that there is a correlation between the residence time and the transcription activity of a certain transcription factor, so maybe understanding for how long a protein stays bound to its promoter can be enlightening about its strength as a transcription activator. Indeed it has been seen that the reason why some mutants are very poor transcription activators is due to the fact that they stay bound to their promoter for a very short time. Taking that into account could be helpful to design some transcription factors which are very strong activators, without needing to modify the motif of the promoter.
References
- Banani, S., Lee, H., Hyman, A. et al. Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol 18, 285–298 (2017).
- Bledsoe, R.K., Montana, V.G., Stanley, T.B., Delves, C.J., Apolito, C.J., McKee, D.D., Consler, T.G., Parks, D.J., Stewart, E.L., Willson, T.M., et al. Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell 110, 93–105 (2002)
- Garcia DA, Johnson TA, Presman DM, et al. An intrinsically disordered region-mediated confinement state contributes to the dynamics and function of transcription factors. Mol Cell. (2021)
- Kent, S., Brown, K., Yang, C.H., Alsaihati, N., Tian, C., Wang, H., and Ren, X. Phase-Separated Transcriptional Condensates Accelerate TargetSearch Process Revealed by Live-Cell Single-Molecule Imaging. Cell Rep. 33, 108248 (2020)
- Stortz, M., Pecci, A., Presman, D.M. et al. Unraveling the molecular interactions involved in phase separation of glucocorticoid receptor. BMC Biol 18, 59 (2020)