Does skeletal muscle have an epigenetic memory?
Epigenetic memory of skeletal muscle hypertrophy was found in a group of men subjected to an experimental program.
Recently, a group of researchers from the United Kingdom undertook a study aimed at the identification of possible epigenetic memory in skeletal muscle by hypertrophy [1].
Their aim was to know how the muscle was influenced by induced environmental stimuli. They also studied how muscle hypetrophy influenced gene expression to get more information or details in the hope of identification useful therapies to improve the quality of life in situations where muscular regression is present such as senescence [2], injuries, and pathologies. To this end the researchers subjected a group of male individuals to a period of experimentation by monitoring the muscular conditions on three phases : an initial period of resistance training, followed by a period of absolute rest, followed by a second training period. Then the y measured the presence of an epigenetic memory induced by muscle hypertrophy. What is epigenetic memory? How much and how is it going to be useful for future generations to have a control on it?
Epigenetic memory means the ability of the tissue to respond differently to environmental stimuli, in an adaptive or maladaptive way. It has been clear for many years that there is an epigenetic memory, which is influenced by the surrounding environment and it can stimulate a positive gene expression (muscle growth and healthy maintenance for life) or negative (loss muscle in old age). The starting point of the British researchers was based on the scientific evidence showing the consequences of a previous exposure to negative environmental stimuli and a consequent epigenetic response modification, which has been “remembered” over time. One of the most influential modifications in epigenetic regulation is the methylation of particular CpG DNA sites; through the methylation or demethylation of these areas it is possible, respectively, to induce expression or repression of the genes closed into these. To confirm this evidence, there are several studies, including the one carried out on individuals born in the period of the Dutch famine of 1943. These individuals showed various pathologies arised from malnutrition in the prenatal period including cardiovascular diseases, hypertension, obesity, raised lipids and reduced glucose tolerance in adult life. Subsequent analysis of the CpG sites of these individuals has been noted an excessive methylation of DNA, which has led to a correlation with the occurrence of these [3]. Further examples of studies, performed on mice, showed how a status of reduced caloric intake in the nutrition of mothers, led to a reduction in the number of muscle fibers in the progeny, correlated to a high methylation of CpG sites[4]. Therefore, if these hypermethylations are related with negative environmental events, the research group assumed that positive environmental events could induce hypomethylation. In particular, the researchers decided to undertake studies to verify this hypothesis, starting from the study about human skeletal muscle. The experiment, which involved the participation of 8 male individuals, consisted on submitting them to 7 weeks of intense muscular effort, followed by 7 weeks of absolute rest to conclude with other 7 weeks of muscle reloading, all preceded by a week of familiarization with the exercises. From the analysis of the samples of muscle tissue, obtained through biopsies carried out immediately after the end of each of the 7 weeks, the researchers obtained results, highlighting particular gene clusters that showed interesting activities following the activity carried out during the experimentation. In particular, two clusters, named as CLUSTER A and CLUSTER B, showed methylations of the respective CpG sites correlated with what we define as epigenetic memory. Cluster A was hypomethylated, compared to baseline, after the loading phase, which corresponds to an increase in gene expression; it was re-methylated during the rest period where has been noticed a decrease in gene expression and finally it was hypomethylated after reloading with consequent gene overexpression. Cluster B also showed a higher hypomethyl status in the first 500 CpG sites than other ones as a consequence of load-induced hypertrophy. As in the case of Cluster A, Cluster B genes were methylated at baseline and have become hypomethylated after initial loading. Unlike Cluster A, Cluster B remained hypomethylated with the discharge, even when the muscle returned to baseline levels, and this hypomethylation was recalled after the induced hypertrophy by recharging (Figure 2).
The British researchers have therefore shown that epigenetic memory is present in muscle hypertrophy induced by a previously encountered ‘load’, as is accurately described in the data reported in the publication[1].
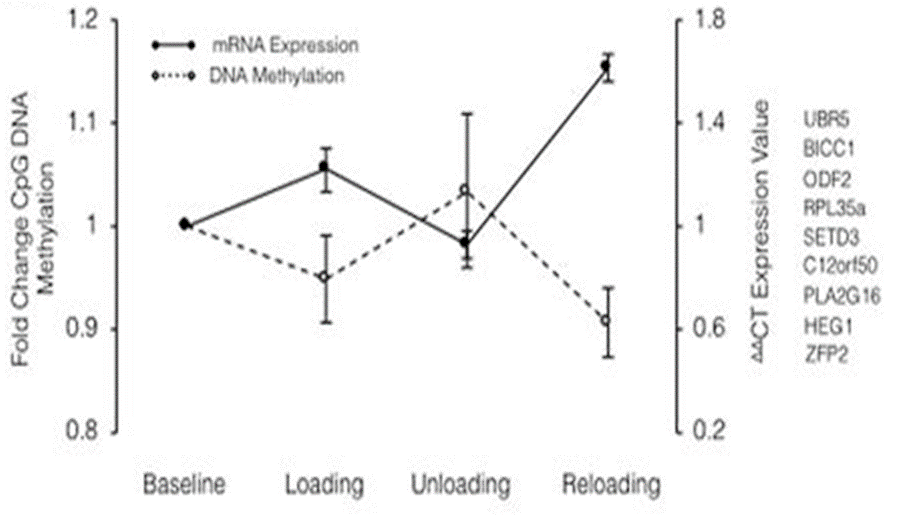
Figura 1: CLUSTER A
CLUSTER A GENI: UBR5,BICC1,ODF2 ,RPL35a, SETD3,C12orf50,PLA2G16,HEG1,ZFP2.
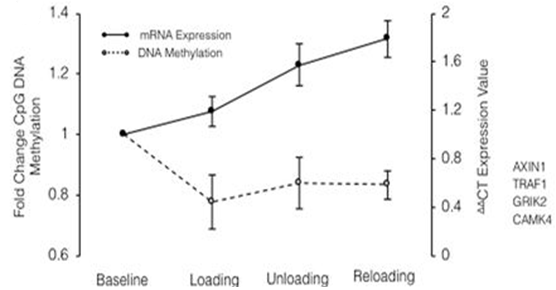
Figura 2: CLUSTER B
CLUSTER B GENI: AXIN1, GRIK2, CAMK4, TRAF1
In our opinion, some aspects of fundamental importance have been omitted. It must be underlined that the participants in the trial were subjects, not previously trained and had only 7 days to familiarize themselves with the exercises before the start of the 21 weeks of experimentation. It is therefore necessary to underline that during the third phase of the experiment, that is the recharge, the participants had acquired a level of experience, to this particular training, better than the first phase of charge. The subjects could therefore have had different gene expressions due to various exercises. Due to that, we believe that carrying out a second part of the experiment by repeating the unloading phase and a new recharge phase would be fundamental to assess whether the results obtained previously were not affected by errors related to the training capacity of the group of participants, moreover, it would be available more data which would provide important information able to give further confirmation of the results or to refuse them. Another point that has not been mentioned in this article is the eating plan that the participants followed during the 21 weeks, since it is known that nutrition greatly influences muscle growth practicing a physical effort like the one practiced by the participants[5,6]. We also believe that research should further consider the lifestyle of the participants, reporting data on the daily lives of these subjects which, being different from each other, may have different stress values and therefore may have altered some research results7-8. In conclusion, we believe that the study is quite reliable, but certainly needs further evidence to confirm the thesis exposed by the researchers.
References:
- Human Skeletal Muscle Possesses an Epigenetic Memory of Hypertrophy. Seaborne RA, Strauss J, Cocks M, Shepherd S, O’Brien TD, van Someren KA, Bell PG, Murgatroyd C, Morton JP, Stewart CE, Sharples AP. PMID: 29382913
- Exercise Promotes Healthy Aging of Skeletal Muscle. Cartee GD, Hepple RT, Bamman MM, Zierath JR. Cell Metab. 2016 Jun 14;23(6):1034-1047. doi: 10.1016/j.cmet.2016.05.007. Review. PMID: 27304505
- Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental. Rudolf Jaenisch & Adrian Bird Nature Genetics volume 33, pages 245–254 (2003)
- Developmental Influences, Muscle Morphology, and Sarcopenia in Community-Dwelling Older Men P. Patel K. A. Jameson H. E. Syddall H. J. Martin C. E. Stewart C. Cooper A. A. Sayer The Journals of Gerontology: Series A, Volume 67A, Issue 1, 1 January 2012, Pages 82–87.
- How nutrition and exercise maintain the human musculoskeletal mass Henning Wackerhage and Michael J Rennie PMCID: PMC2100208 PMID: 16637871
- Early diet and growth: impact on ageing. Sayer AA1, Cooper C. Proc Nutr Soc. 2002 Feb;61(1):79-85.
- Social environmental effects on gene regulation – Jenny Tung, Yoav Gilad, Cell Mol Life Sci. 2013; 70(22): 4323–4339. Published online 2013 May 18. doi: 10.1007/s00018 013-1357-6, PMCID: PMC3809334.
- Environmental Influences on Gene Expression – Ingrid Lobo, Ph.D. (Write Science Right) © 2008 Nature Education Citation: Lobo, I. (2008)Environmental influences on gene expression. Nature Education 1(1):39
