Epigenetic regulation of bivalent chromatin in cranial neural crest cells
Abstract
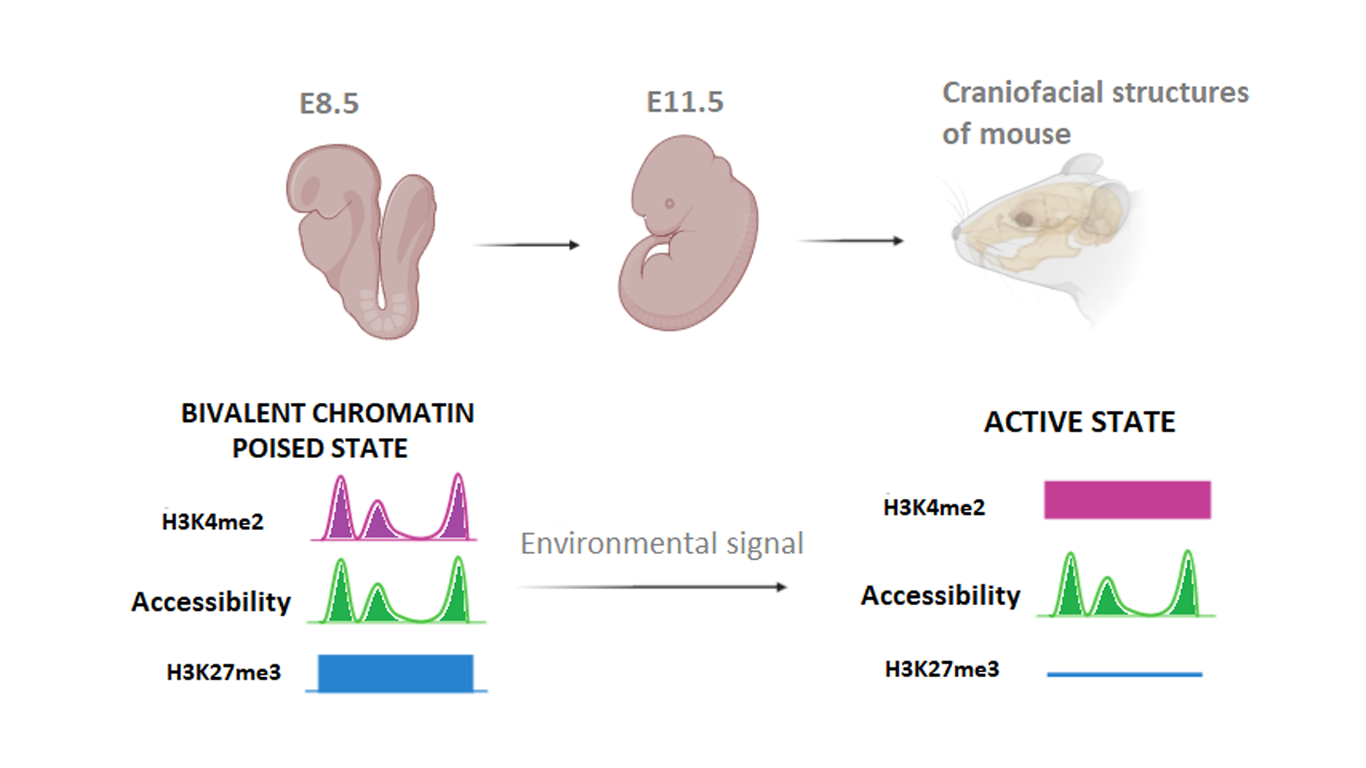
The epigenetic regulation of cranial neural crest cells is fundamental to maintain positional plasticity through migration and to have a normal craniofacial development. Bivalent chromatin plays a main role in this regulation.
Cranial neural crest (NC) cells are central to craniofacial development. NC cells are multipotent with migratory properties. They detach from the neuroepithelium of the dorsal neural tube and migrate along a certain number of defined pathways, differentiating into different cell types (neurons, glia, melanocytes), but also into chondrocytes and osteoblasts contributing to the formation of bone and cartilaginous elements of cranial skeleton [1]. Each population of NC cells can acquire a positional identity and the epigenetic regulation of chromatin influences these processes allowing to maintain a broad competence through migration.
Minoux and collaborators [2] studied 4 subpopulations of NC cells: frontonasal, maxillary, mandibular and second pharyngeal arch of E10.5 mouse embryos through the use of 4 techniques: FACS to isolate cells of each subpopulation; RNA-seq to study transcriptional profiles; ChIP-seq to analyze chromatin profiles and ATAC-seq to highlight chromatin accessibility.
The skeletogenic cephalic neural crest cells derive from two domains of the brain: one anterior in which the NC cells do not express Hox genes (Hox-negative domain) that forms the head and facial skeleton and one posterior in which NC cells express Hox genes (Hox-positive domain) that forms hyoid cartilage [4].
The researchers identified more than 3000 genes differentially expressed between subpopulations by RNA-seq of which 237 are transcription factors. They next analyzed chromatin profiles of each NC cells subpopulation by ChIP-seq. In particular, they studied histone modifications such as Polycomb group (PcG)-dependent repressive H3K27me3 and activation modifications as H3K27ac and H3K4me2.
PcG proteins are part of a large family that epigenetically repress the transcription of important genes for development. They form 3 broad enzymatic complexes called Polycomb Repressive Complexes (PRC), each of which is able to modify chromatin through different mechanisms, but in this case it is significant the activity of PRC2. PRC2 is a methyltransferase that catalyze the mono-, di- and tri-methylation of H3K27 thanks to the catalytic domain of Ezh2 [3].
Chromatin profiles of promoters and enhancers show that H3K27me3 marks correspond to nonexpressed genes while H3K27ac marks correspond to highly expressed genes in each subpopulation. The promoters and enhancers of genes, taken into consideration, were enriched in a different way in each subpopulations. For instance, the locus of Hoxa2 was marked by H3K27ac and H3K27me3, respectively in second pharyngeal arch and frontonasal, maxillary and mandibular NC cells.
Chromatin profiles of promoters and enhancers show that H3K27me3 marks correspond to nonexpressed genes while H3K27ac marks correspond to highly expressed genes in each subpopulation. The promoters and enhancers of genes, taken into consideration, were enriched in a different way in each subpopulations. For instance, the locus of Hoxa2 was marked by H3K27ac and H3K27me3, respectively in second pharyngeal arch and frontonasal, maxillary and mandibular NC cells. A large group of promoters and enhancers showed both marks H3K27me3 and H3K4me2, confirming the presence of bivalent chromatin. This bivalence maintains genes in a transcriptionally silenced state while keeping them ready to activation.
These data have been expanded with ATAC-seq analysis, which showed a correlation between chromatin accessibility and bivalent chromatin in transcriptionally inactive genes. The epigenetic switch from H3K27me3 to H3K27ac at bivalent promoters and enhancers contributes to activate genes with gain of H3K27ac and loss of H3K27me3. Consequently, these histone modifications are involved in transcriptional regulation of fundamental genes for development. Therefore, RNA changes are better explained by different enrichment of histone modifications at bivalent promoters and enhancers, rather than by chromatin accessibility.
All these data were compared with Ezh2cKO mutant to study the key role of Ezh2 in PRC2. The researchers analyzed transcriptional state, histone modifications, and chromatin accessibility of each subpopulations of mutant. Ezh2cKO mutant embryos showed normal cranial NC cell migration until at least E10.5, but at E18.5 most of the craniofacial structures were absent. The authors also studied histone modifications in mutant embryos and compared these data with WT NC cells (control). H3K27me3 was absent in Ezh2cKO NC cells, just to confirm the catalytic function of Ezh2 in PRC2 about trimethylation of histone.
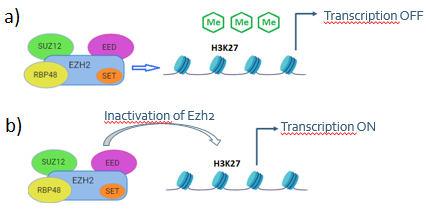
Figure 1 – a) Catalytic function of Ezh2 in PRC2 in WT NC cells. b) Inactivation of Ezh2 (Ezh2cKO mutant).
Moreover, the up-regulated genes in Ezh2cKO had bivalent chromatin in WT cranial NC cells, while promoters of genes enriched only with H3K27me3 or H3K4me2 in control, remained unchanged in Ezh2cKO. Ezh2cKO NC cells partially lost their positional identities because subpopulations segregated less from each other than WT subpopulations. In addition, a selective gain of H3K4me2 was found in the Polycomb domains compared to sites of high H3K27me3 enrichment in WT cells. Consequently, the long sites of high level H3K27me3 in Polycomb domains could serve as a mechanism to oppose the deposition of H3K4me2 at accessible sites.
In conclusion, it is stimulating to analyze the specification of positional identities of cranial NC cells, in particular the organization of chromatin and epigenetic regulation of these processes in vivo. In my opinion, it would be interesting to study the role of Ezh1 in addition to Ezh2.
References
- Le Douarin N.M., Creuzet S., Couly G., Dupin E. Neural crest cell plasticity and its limits. Development (2004) 131, 4637–4650 doi: 10.1242/dev.01350;
- Minoux M., Holwerda S., Vitobello A., Kitazawa T., Kohler H., Stadler M.B., Rijli F.M. Gene bivalency at Polycomb domain regulates cranial neural crest positional identity. Science (2017) 355, eaal2913 doi: 10.1126/ science.aal2913;
- Chittock E.C., Latwiel S., Miller T.C.R., Muller C.W. Molecular architecture of polycomb repressive complexes. Biochemical Society Transactions (2017) 45, 193–205 doi: 10.1042/BST20160173;
- Creuzet S., Couly G., Vincent C., Le Douarin N.M. Negative effect of Hox gene expression on the development of the neural crest-derived facial skeleton. Development (2002) 129, 4301–4313;