Gapmer Antisense Oligonucleotides: a turning point in the treatment of Ullrich Congenital Muscular Dystrophy?
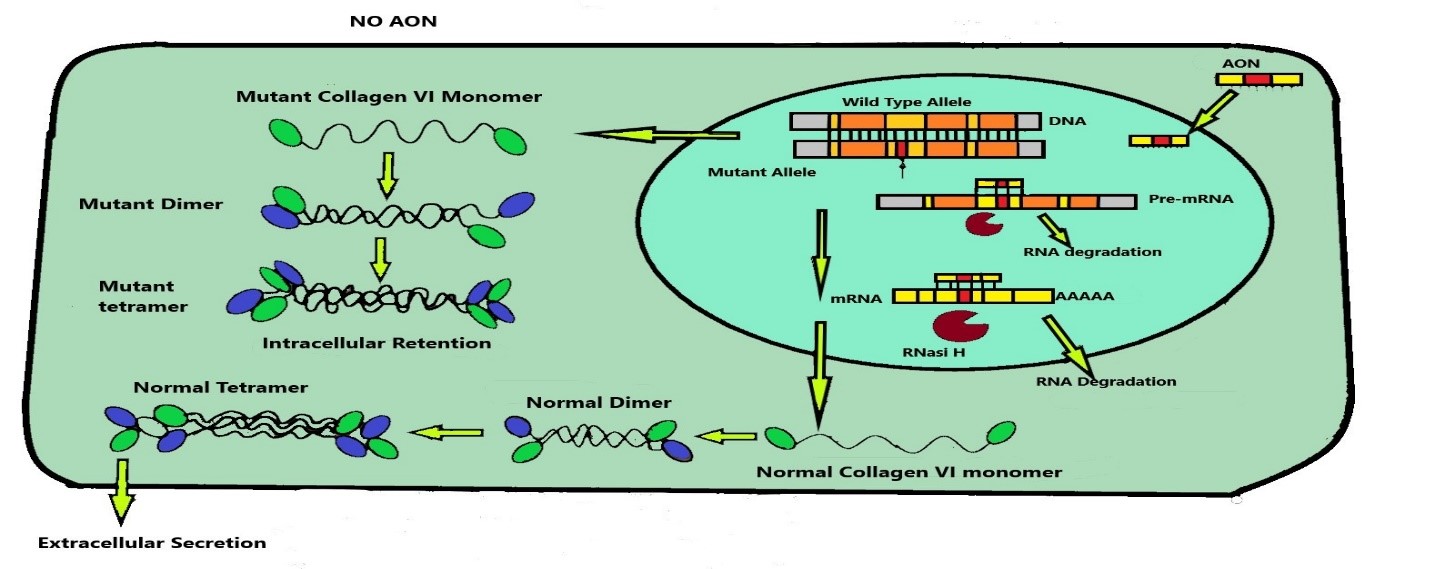
Figure 1: A schematic representation of COL6A3 wild type and mutant allele expression in presence and in absence of gapmer AON.
silencing than AONs targeting pre-mRNA. In particular, they discovered that 22-mer gapmer AONs with 8 to 10 nucleotides had the higher efficiency, with near complete suppression of the mutant transcripts. Because of its strong activity, it could represent a potential drug against UCMD. Next was determinated its effective concentration which has been established at about 20 nM. Lower concentrations didn’t have significant effects, while higher concentrations led to off-target effects. Therefore, they treated dystrophic fibroblasts with a 22-mer gapmer AONs to analyse shape and location of collagen VI after the suppression of mutant allele of COL6A3. They noted an improvement since intracellular collagen VI retention was decreased, while its deposition in ECM was increased. Moreover, the pattern of collagen in the ECM changed to linear fibrils, becoming similar to that of healthy fibroblasts. Thanks to this study these researchers have shown for the first time the development of an allele specific silencing therapy by gapmer AONs to reduce dominant negative mutation effects in fibroblast affected by UCMD. In fact, the application of these antisense oligonucleotides on this fibroblast cellular line suppressed the expression of the mutant allele of COL6A3 and improved fibroblasts general features. However, we can’t say whether this therapy maintains its efficiency when it is given to patient, because it was not tested in vivo. Antisense oligonucleotide are drugs which have to be injected parenterally. After the injection, they circulate in blood bonded to plasma proteins to reach target cells. Their half-life in blood circulation depends by their affinity for plasma protein. Oligonucleotides with high affinity for plasma protein can easily reach target cells, while the ones with low affinity for plasma proteins have to be modified by attaching lipophilic substituents, such as cholesterol, receptors ligands, peptides or PEG. Oligonucleotides are carried into the target cell through two different vescicular pathway: the first results in their release in the cytoplasm, while the second delivers them to the lysosomes. The second pathway is very detrimental, because lysosomal enzymes attack and degrade AONs.
Furthermore, the oligonucleotides exhibit a dose dependent toxicity. The potential toxicities for gapmer antisense oligonucleotides can be classified as hybridization dependent, which occurs when AONs bind an off-target mRNA, and hybridization independent. Clinically, the main toxicity problems have proven to be hybridation independent effects, such as prolongation of partial activation of thromboplastin, infection in the injection site and physiological symptoms, like headache and fever. An other problem related to their use in vivo is due to the abundance of muscles in our body, in fact they constitute 80%. Therefore, it’s very difficult for AONs to reach them all. To solve this problem, it would be necessary to inject very high doses, which, however, would lead to additional toxic effect.
Therefore, the therapy based on gapmer AONs proved to be efficient in mutant COL6A3 gene silencing when it is applied directly to dystrophic fibroblasts, but it can’t be as effective if it is given to patient affected by UCMD owing to the reasons listed above. Accordingly, before any clinical application of this therapy, future studies in vivo are needed in order to evaluate actual functioning of gapmer AONs in allele-specific silencing.
References
- Elena Marrosu, Pierpaolo Ala, Francesco Muntoni and Haiyan Zhou; “Gapmer Antisense Oligonucleotides Suppress the Mutant Allele of COL6A3 and Restore Functional Protein in Ullrich Muscular Dystrophy”; Molecular Therapy: Nucleic Acids Vol. 8 September 2017, 15;8:416-427.
- Frank Bennett and Eric E. Swayze; “RNA Targeting Therapeutics: Molecular Mechanisms of Antisense Oligonucleotides as a Therapeutic Platform”; Annu. Rev. Pharmacol. Toxicol. 2010. 50:259–93.
