Identification of cells capable of regenerating damaged intestine: revival stem cells
Abstract
Following injury, the intestinal epithelium is able to recover its original function and cellular composition. However, little is known about the cell subtypes responsible for initiating the regeneration process. Ayyaz et al. [1] identified an injury-induced quiescent cell type, named revival stem cells (revSCs), that are responsible for epithelium regeneration. Further characterization and understanding of this population may be instrumental to develop novel therapies in acute intestinal injury.
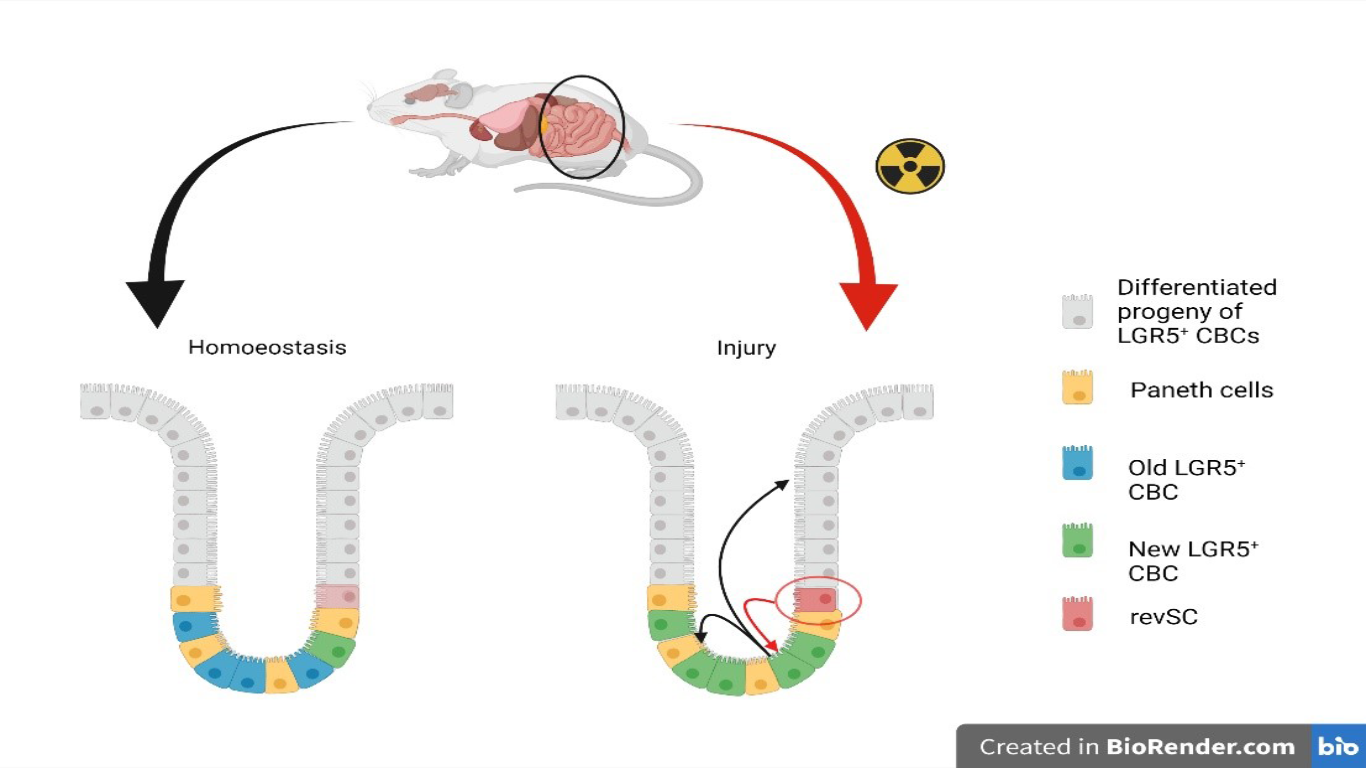
The intestine is the largest organ of the human body and performs several functions that are indispensable for our organism. The structure of the intestinal mucosa is characterized by the villi and the crypts, the latter are invaginations of the epithelial tissue that constitute the functional units of the intestine and ensure epithelial turnover (about 3-5 days). The multipotent columnar cells of the intestinal crypts (CBCs), that drive intestinal epithelium turnover, express Lgr5 marker and generate all progenitors of differentiated intestinal epithelium cells [2]. CBCs can be lost as a result of injury, such as irradiation, but the intestinal epithelium is still able to recover [3]. In a recent study, Ayyaz and collaborators, investigated which cells were able to drive intestinal regeneration following injury. Through single cell RNA sequencing (scRNAseq), mouse intestinal cells were profiled and a distinct damage-induced quiescent cell type was identified: revival stem cells (revSCs).
To define the mechanisms that underlie regeneration, single cells were transcriptionally profiled from mouse small intestine epithelium three days after exposure to 12 Gray radiation [1]. ScRNAseq, performed on whole epithelia and enriched crypts, revealed 17 distinct epithelial clusters and 2 lymphocyte clusters. One of the identified clusters did not express any line-specific markers. To define stem cell dynamics, the signature of CBCs was identified in enriched crypts, which were mapped as SSC1 cluster (single profile stem cell cluster 1). It mostly includes proliferating cells from undamaged crypts that were strongly suppressed following irradiation.
The lineage-specific marker-free cluster SSC2 was then identified, which comprised almost exclusively cells from irradiated crypts and was distinguished by the expression of several genes involved in inflammatory responses, DNA damage responses, and cell survival. Substructure analysis in SSC2 showed three distinct groups: SSC2a, SSC2b, and SSC2c. The signature of the SSC2a subgroup is evident in SSC1, suggesting that SSC2a cells are transcriptionally close to CBCs. Whereas SSC2c cells were found to be a quiescent population with a distinct gene signature including Clusterin (Clu), Anxa1, Cxadr, and Basp1. Notably, high expression of Clu is unique to SSC2c cells and Clu expression has been previously shown to enhance cancer cell survival [4]. Immunofluorescence analysis revealed that Clu+ cells, in homeostatic intestines, are rare.
The transcriptional regulator YAP1 is important for regeneration of the intestinal epithelium [5]; its elimination suppresses the emergence of endogenous Clu+ cells. In addition, Clu+ cells are quiescent, as demonstrated by their low incorporation of the cell proliferation marker EdU. SSC2c Clu+ cells are, therefore, rare, unique, quiescent crypt cells induced by intestinal damage in a Yap1-dependent manner [1]. To demonstrate that Clu+ SSC2c cells are important for regeneration they were labelled together with their progeny in ClutdTomato mice following irradiation. Clu+ daughter cells contributed to Lgr5+ CBCs, therefore Clu+ SSC2c cells give rise to a de novo Lgr5+ CBC population. In addition, Clu+ cells also give rise to progeny of differentiated cells with a precise temporal hierarchy. The overall results demonstrated that Clu+ SSC2c cells reconstitute Lgr5+ cells to revive crypts. These cells have been called revSCs.
The functional importance of these Clu+ revSCs was determinated in CluiDTA mice, in which Clu+ cells are ablated by TAM treatment. After irradiation, revSC-deficient intestinal epithelia (Cluless) showed greatly reduced proliferation, crypt number, and crypt length, and the colon was shortened. Collectively, Ayyaz and his collaborators identified revSCs as a cell type that occurs in the damaged intestine according to a Yap1-dependent signal to reconstitute Lgr5+ CBCs and regenerate the intestine.
In conclusion, damage-induced expansion of revSCs may, therefore, be a key mechanism that underlies tissue repair in response to injury.
We are pleasantly surprised by this work, because the authors identified for the first time, through Clu expression, a population of damage-induced quiescent cells capable of regenerating the injured epithelium. Previous studies had tried to identify the population through a different gene signatures but the experiments had not shown convincing results. This new discovery could be extremely important and the use of revSCs could have several applications as therapy in the case of acute intestinal injury.
References
- Arshad Ayyaz, Sandeep Kumar, Bruno Sangiorgi, Bibaswan Ghoshal, Jessica Gosio, Shaida Ouladan, Mardi Fink1, Seda Barutcu, Daniel trcka, Jess Shen, Kin Chan1, Jeffrey Wrana & Alex Gregorieff. Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature. 2019.
- Laura R McCabe, and Narayanan Parameswaran. Recent Advances in Intestinal Stem Cells. Curr Mol Biol Rep. 2017 Sep; 3(3): 143–148.
- Metcalfe, , Kljavin, N. M., Ybarra, R. & de Sauvage, F. J. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell. 2014.
- Zhang,F. et Clusterin facilitates stress-induced lipidation of LC3 and autophagosome biogenesis to enhance cancer cell survival. Nat. Commun. 2014.
- Gregorieff, , Liu, Y., Inanlou, M. R., Khomchuk, Y. & Wrana, J. L. Yap-dependent reprogramming of Lgr5+ stem cells drives intestinal regeneration and cancer. Nature. 2015.