Industrial application of a new optogenetic gene circuit: OptoEXP-OptoINVRT
An innovative application of metabolic engineering, using optogenetics gene circuit, to optimize the production of industrial products in Saccharomyces Cerevisiae.
Metabolic engineering is a commonly used technique used to modify endogenous metabolic pathways to optimize the yield of interesting products. One of its applications is optogenetics which uses the light to activate the genetic expression, in a nontoxic way.
José L. Avalos and his group at Princeton University, New Jersey, choose optogenetics for its fast pathway regulation to raise the yields of lactate, isobutanol, and 2-methyl-1-butanol in Saccharomyces Cerevisiae [1]. Their production competes with the ethanol synthesis which cannot be completely deleted since it is directly involved in yeast growth. They created a gene circuit activated by blue light (450 nm), called OptoEXP, and a gene circuit repressed by blue light (450 nm), called OptoINVRT. This has laid the condition for experimentation of a nontoxic on-off genetic control that allows a temporal division between the growth phase and the production phase. To obtain a light controlled gene expression a light-sensitive transcription factor of Erythobacter Litoralis was used: EL222 [2]. EL222 specifically binds its promotor PC120 after light stimulation and therefore conformational changes, allowing the gene transcription [3].
The first step to creating a light-activated gene circuit was to fuse EL222 with VP16, a transcriptional activation domain. OptoEXP is the genic circuit in which the VP16-EL222 construct is expressed under the control of PPGK1, a strong constitutive promoter, and in which PDC1, the first enzyme of the ethanol pathway, is expressed under PC120 (Figure 1).
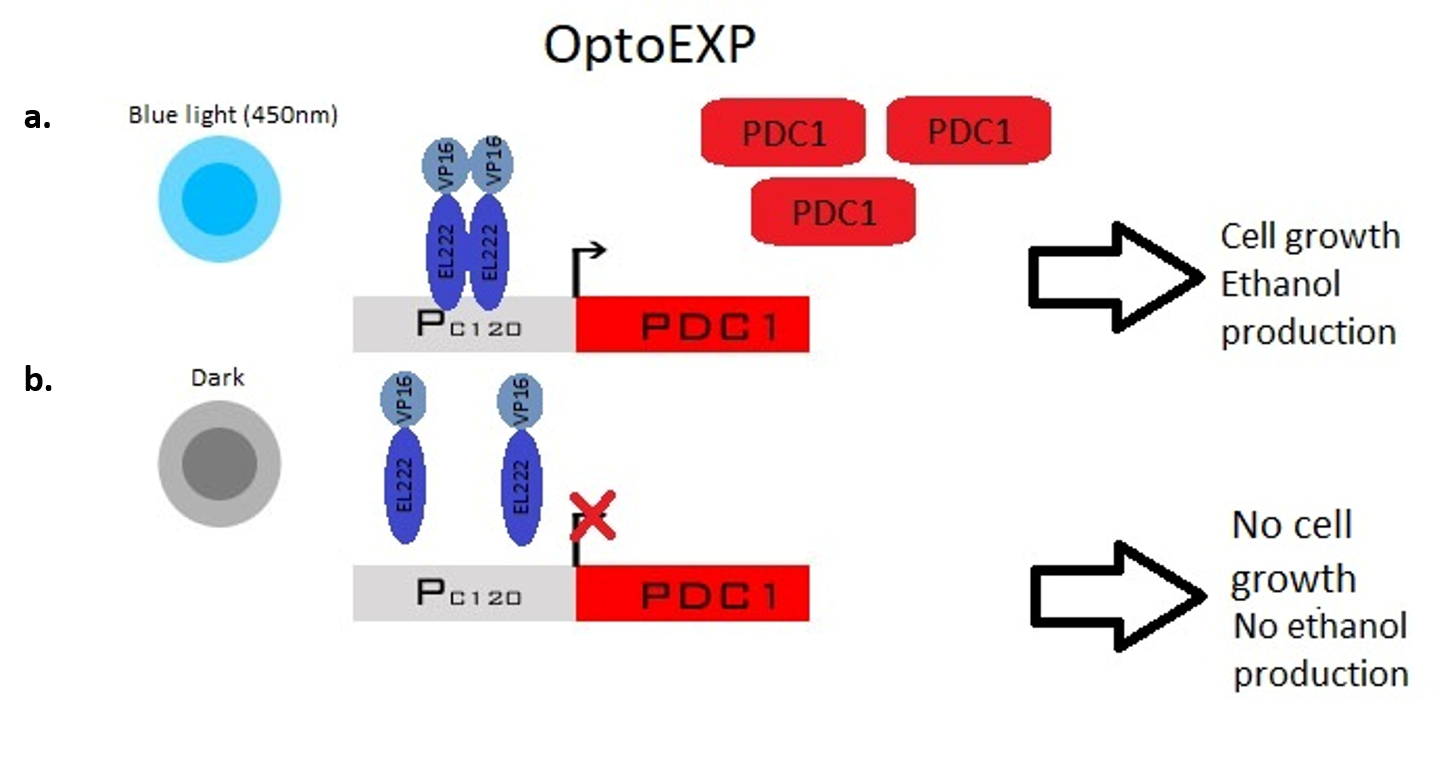
Fig 1. OptoEXP gene circuit. a) light stimulated EL222-VP16 dimerization with consequent PDC1 transcription. b) no dimerization of EL222-VP16
Instead, the light repressed circuit is based on Gal80-Gal4 interaction: Gal80 binds and inhibits the transcription factor Gal4, which, in this way, cannot bind its corresponding promotor Gal1. OptoINVRT is the genic circuit in which Gal80 expression is controlled by PC120, Gal4 is controlled by a strong constitutive promoter, and the desired enzyme is expressed under the control of PGal1. Wisely the authors choose to test three different constitutive promotors for Gal4 creating OptoINVRT1, OptoINVRT2 and OptoINVRT3 circuits. In this way, they saw that OptoINVRT1 guarantees both the best Gal4 expression and Gal4 control (Figure 2).
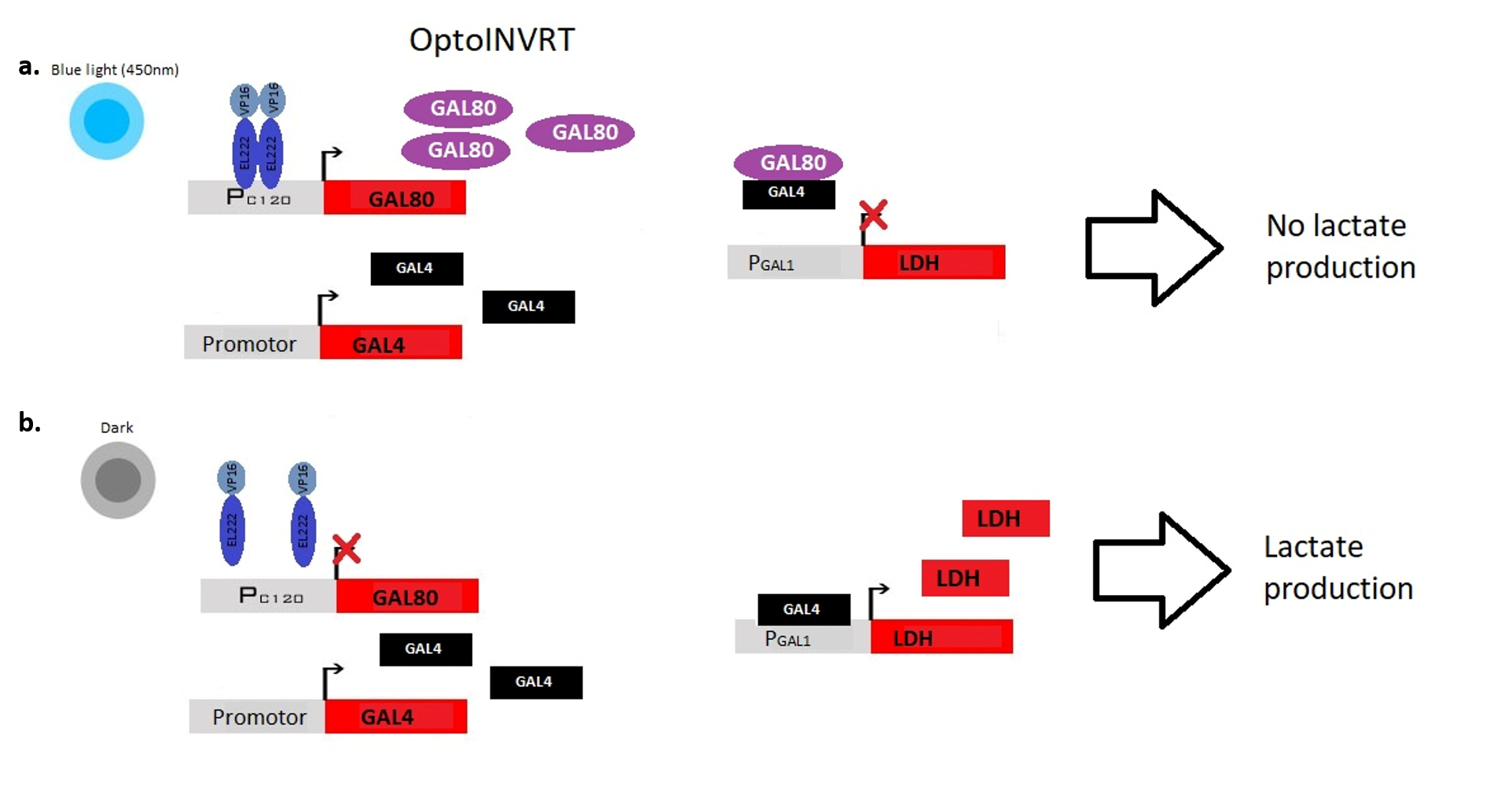
Fig. 2. OptoINVRT gene circuit. a) light stimulated Gal80 transcription with consequent Gal4 inhibition and no lactate production. b) Gal4 transcription in darkness with consequent bound to the Gal1 promotor and lactate production
To see whether the OptoEXP-OptoINVRT system would have an application in industry, it was applicated on the easiest endogenous pathway of Saccharomyces Cerevisiae: the lactate synthesis. In this experiment, the PDC1 gene was regulated by OptoEXP while LDH1 was regulated by OptoINVRT in a yeast strain KO for PDC1, PDC5, PDC6, and LDH1. All three OptoINVRT constructs were tested with a light pattern in which the growth phase was carried for 16h in light and the production phase was carried for 54h in darkness. OptoINVRT1 showed the best light-induced control related to the best lactate yield. The authors also applied the OptoEXP-OptoINVRT system to isobutanol production in yeast strain with ΔBAT1 to further increase both isobutanol and in 2-methyl-1-butanol yield. 2-methyl-1-butanol is a byproduct of the mitochondrial isobutanol synthesis [4]. The genic circuit utilized is composed by the OptoEXP circuit controlling PDC1 and OptoINVRT controlling ILV2. The promising results convinced the authors that optogenetics could be a valid metabolic engineering technique to apply in bioreactors.
First, it was verified whether the light could stimulate the growth phase of the modified yeast strain to match the cell concentration of wild type, in a 2 litres bioreactor. Since the results were positive, the authors proceeded throughout the experiment in a 0,5 litres bioreactor to allow firstly growth and secondly production phase.
Wisely the authors made a series of experiment to select both the best cell concentration in which stop the growth phase and the best period of time to sustain the incubation in darkness that guaranteed the highest production level. Surprisingly the product yield did not rise as expected after the increase of glucose concentration in the medium and the increase of the fermentation time. The authors theorized a metabolic stop due to NAD+ depletion caused by a long period of PDC1 inactivity. Although the experiments to verify this hypothesis needs to be better investigated. To solve this problem, they tried to promote the production phase with light pulses of 30 minutes (15 sec on, 65 sec off) rather than in complete darkness. As a result, they observed a drastic increment in yields of the interesting products.
It remains to be demonstrated that using the new gene circuits to obtain yeast strains is indeed competitive with respect to the currently used methods as it is important to evaluate whether the cost of a translucent bioreactor and the cost of illumination are repaid by the increased yield.
References
- M. Zhao, Evan & Zhang, Yanfei & Mehl, Justin & Park, Helen & A. Lalwani, Makoto & Toettcher, Jared & L. Avalos, Josè. Optogenetic regulation of engineered cellular metabolism for microbial chemical production. Nature. 555. 10.1038/nature26141 (2018)
- . Nash, A. I. et al. Structural basis of photosensitivity in a bacterial light-oxygen voltage/helix-turn-helix (LOV-HTH) DNA-binding protein. Proc. Natl Acad. Sci. USA 108, 9449–9454 (2012)
- Rivera-Cancel, G., Motta-Mena, L. B. & Gardner, K. H. Identification of natural and artificial DNA substrates for light-activated LOV-HTH transcription factor EL222. Biochemistry 51, 10024–10034 (2012).
