Metabolic adaptation to diet: the role of promoter-enhancer interactions
Abstract
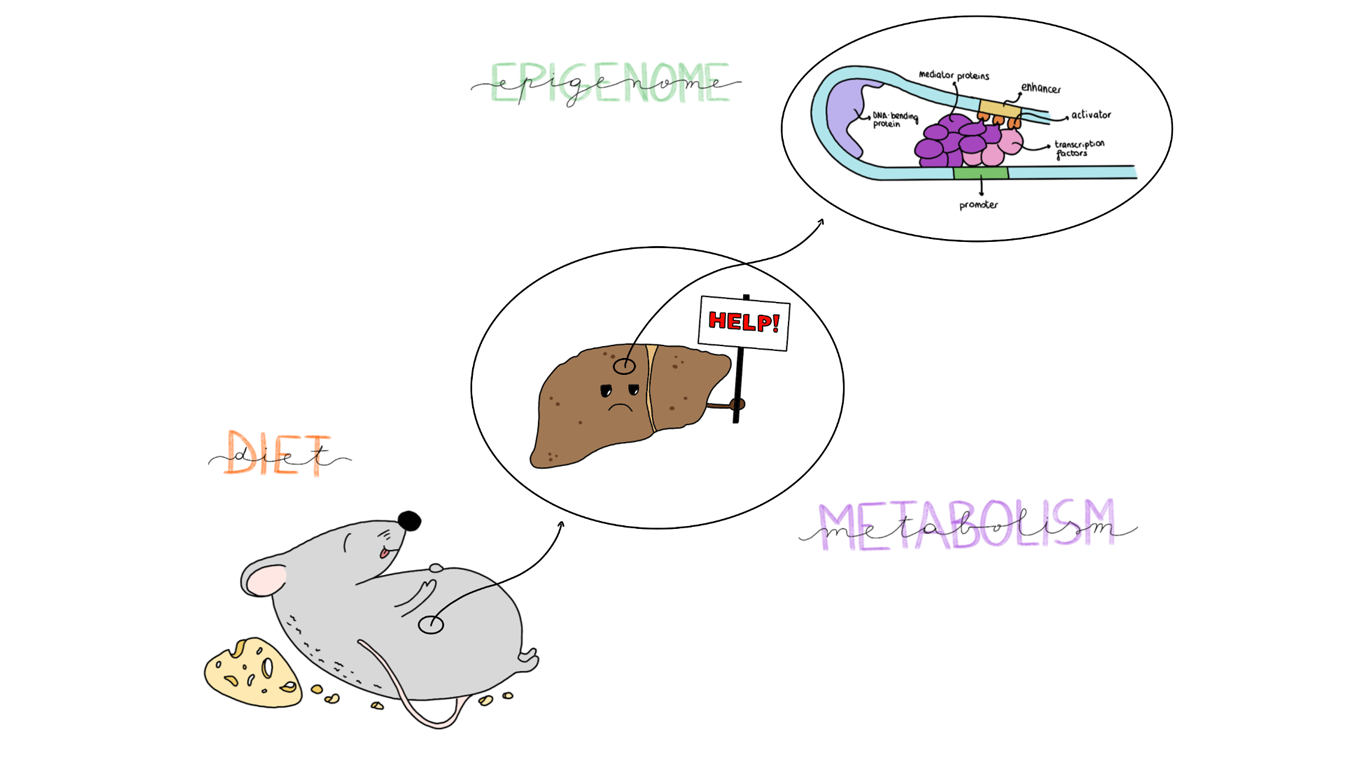
Metabolic diseases are rising with the spread of Western lifestyle and are becoming an emerging health concern. The consumption of an energy rich diet leads to important changes in metabolism on a DNA level: organs, in particular the liver, adapt to overnutrition by modifying their epigenome. A recent study by Qin et al. [1] investigates the role of promoter-enhancer interaction dynamics to provide insights into the mechanism that regulates the progression from metabolic adaptation to disease.
The contemporary epidemics of unhealthy dietary pattern and sedentary lifestyle are linked to the increase in prevalence of metabolic diseases, including non‐alcoholic fatty liver disease (NAFLD) [2]. This pathologic condition is characterized by the build-up of extra fat in liver cells caused by a high-calorie alimentary regimen. Overabundance of nutrients leads to a physiological state that has different homeostatic requirements, which are accomplished thanks to a variety of mechanisms that respond to fluctuations in food intake. Often, a core component of such responses includes alterations in gene expression in key tissues. The target genes are usually involved in metabolic pathways, such as de novo lipogenesis (DNL) or fatty acid oxidation (FAO). Diet can reprogram the epigenome and gene expression by activating enhancers, distal regulatory elements that physically interact with their target gene’s promoter via transcription factors (TFs), leading to the formation of a chromatin loop [3, 4].
To understand how the chromatin organization contributes in regulating gene expression during metabolic adaptation to diet, Qin et al. analysed the livers of male C57BL/6 mice fed with two different diets: a carbohydrate-rich diet (CD) and a lipid-rich diet (LD). After 20 weeks of treatment, animals on the LD became markedly obese and showed poor glucose and insulin tolerance, along with a significant increase in plasma concentrations of cholesterol, high-density lipoprotein and low-density lipoprotein. Through histological analysis, authors measured an increase in adipose tissue mass, liver mass and adipocyte size, especially in the LD group. These multi-system changes reflect adaptation of metabolism and physiology of the animal to an extreme diet. RNA-seq and differentially expressed gene (DEG) analysis confirmed that this adaptation occurs through a reprogramming of the transcriptome: in fact, each diet showed a set of upregulated and downregulated genes. In particular, the LD group showed an upregulation of genes involved in FAO and a downregulation of genes involved in DNL.
Promoter capture Hi-C (PCHi-C) data showed that more than half of cis-interactions is shared across conditions and can then be considered stable. On the contrary, promoter-enhancer interactions that change depending on diet can be divided into two categories: newly formed and preformed loops.
Starting with newly formed promoter interactions, researchers used a software to find altered promoter looping events. Notably, they identified more rewired interactions in animals on CD rather than in animals on LD. In addition, only the promoter-enhancer interactions responsive to CD significantly associated with increases in gene expression. These results suggest that adaptation to a carbohydrate-rich diet leads to de novo formation of promoter-enhancer interactions.
The authors then examined the overlap of promoter-enhancer interactions with H3K27ac differentially enriched loci, in order to understand whether the activity status of the enhancers in preformed interactions reflected gene expression. Profiling of genomic H3K27ac by ChIP-seq revealed about 5000 loci with significantly differential enriched signal induced by diet. These loci were mostly located far from the transcription start site, and they were classified as enhancers. DEGs’ promoters interacting with an acetylated enhancer were upregulated, regardless of diet. Remarkably, the number of promoter-enhancer loops in which enhancer’s activation status changes because of diet greatly exceeds the number of new loops. These findings suggest that activation of enhancers already interacting with a promoter represents an important modality of gene regulation during metabolic stress, in particular in the LD group.
In contrast to differential promoter-enhancer loops, higher-order chromatin organization, such as topologically associating domains (TADs), is largely unchanged by diet, as confirmed by Hi-C analysis.
To evaluate which transcription factors are involved in gene expression changes, researchers used HOMER on DEGs’ promoters and observed an enrichment in Hnf4α motifs. ChIP-seq revealed more Hnf4α enriched peaks in animals on LD, indicating that Hnf4α has an important role in regulating lipid metabolism. In addition, Hnf4α binding was higher at acetylated enhancers. PCHi-C and Hnf4α ChIP-seq data showed that promoter-enhancer interactions overlapped with Hnf4α binding. DEGs that showed gains in Hnf4α peaks at the promoter-enhancer interactions also showed upregulated gene expression.
Since only 10% of the genome-wide binding sites for Hnf4α changed by diet, authors hypothesized that Hnf4α may interact with other transcription factors during metabolic stress. HOMER analysis of Hnf4α binding regions showed that the second most common motif is the one for the basic leucine zipper domain (bZIP) family of transcription factors, which includes C/EBPα. C/EBPα ChIP-seq showed that more than 20% of C/EBPα binding sites changed by different diets and 55% of the peaks colocalized with Hnf4α in liver. When Hnf4α is present, C/EBPα binding signals increase at diet-induced C/EBPα binding sites in both conditions. These data suggest that the two TFs work together to regulate gene expression during metabolic adaptation to diet.
In conclusion, this study offers a deeper understanding in how chromatin interactions and gene expression respond to metabolic and physiologic stress and demonstrates that an unhealthy dietary pattern and a sedentary lifestyle can modify the epigenome, affecting health state on a molecular scale. In addition, it associates NAFLD, an emerging metabolism-based pathology, with diet and gene expression in mice. Further studies on human livers could help in finding the differences between the two models and identifying possible therapeutic targets.
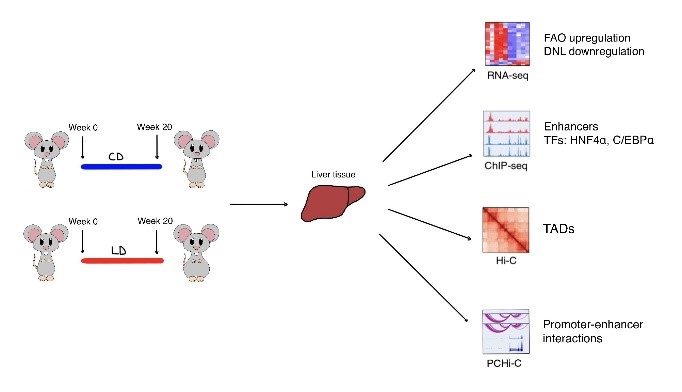
Fig. 1. Schematic overview of the study. Mice have been fed with two different diets for 20 weeks and their liver tissues were analysed using four techniques: RNA-seq, ChIP-seq, Hi-C and PCHi-C.
References
- Qin, Y. et al., Alterations in promoter interaction landscape and transcriptional network underlying metabolic adaptation to diet, Commun., 11(2020): 962.
- Al-Dayyat, H. M. et al., Non-alcoholic fatty liver disease and associated dietary and lifestyle risk factors, Diabetes Metab. Syndr., 12(2018): 569-575.
- Siersbaek, M. et al., High fat diet-induced changes of mouse hepatic transcription and enhancer activity can be reversed by subsequent weight loss, Rep., 7(2017): 40220.
- Qin, Y. et al., An obesity-associated gut microbiome reprograms the intestinal epigenome and leads to altered colonic gene expression, Genome Biol., 19(2018): 7.