Rheumatoid Arthritis: Epigenetic factors
Prevalence of circulating immunocomplexes (ICs) strongly correlates with rheumatoid arthritis (RA) in humans: IgG immunocomplexes sensitize human monocytes for inflammatory hyperactivity via epigenetic reprogramming.
The production of autoantibodies is the hallmark of systemic autoimmune disorders such as Rheumatoid Arthritis, a chronic inflammatory disease of the joints. This is a multifactorial pathology [1]. In fact, among the causes so far listed we find environmental origin, lifestyle, and genetics. They have taken IgG-ICs precipitated from sera of RA patients and IgG-ICs from sera of healthy controls, put them in contact with human blood monocytes in vitro and compared the results. In the first case, it has been detected an inflammatory functional state, characterized by a strong secretion of inflammatory cytokines (TNF-a, IL-1b, IL-6 and IL-8) in response to a variety of innate stimuli [2], including damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs). This study provides a detail molecular mechanism for this phenomenon. The interaction between IgG-ICs and its receptor on the monocyte’s surface recruits two transcription factors that bind promoter of genes encoding inflammatory cytokines, recruits also a histone methyl-transferase: the SETD3.
The starting point of this work was the analysis of SETD3 expression in monocytes interacting with cIgG (Mo-cIgG) vs not interacting monocytes (Mo RPMI). Taking into account the increased expression in Mo-cIgG, the catalytic activity of SETD3 was quantified, by measuring the percentage of methylation in the inflammatory cytokines’ genes. Indeed, the Mo-cIgG interaction induces the activation of methyltransferase and its catalytic activity is increased. Once this has been established, it has been interesting to analyse whether it actually increases the transcription of genes leading to the inflammatory phenotype in Mo-cIg after contact with an exogenous antigen.
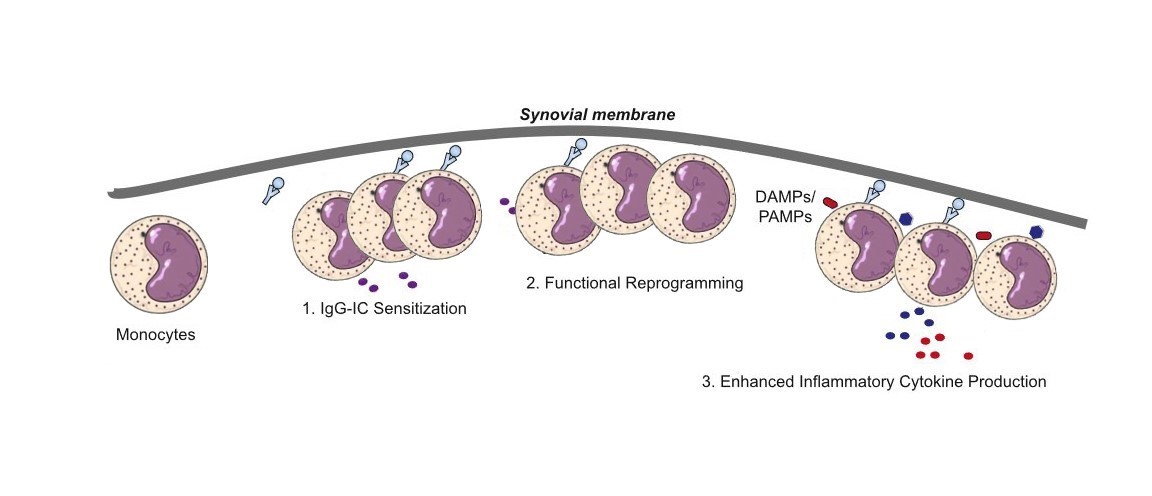
Figure 1 Hypothesis on the molecular mechanisms of IgG-IC–mediated hyperactivity training of monocytes/macrophages. Monocyte/ macrophage functional programming is triggered by IgG-IC binding to FcγRIIa
As in the previous experiments, the results obtained were encouraging. The last step to complete the study was to verify if this mechanism and pathway studied in vitro could be found in the human synovia of RA patients. They compared the two signatures gene expression of the Mo-IgG sensitized in vitro with the Mo-RA patients. Intriguingly, was found a quite good similarity between the two signatures. These results lead to an advanced understanding of the molecular mechanism of this pervasive immunological event but also indicates potential targets for therapeutic intervention of a range of key inflammatory autoimmune diseases.
Equally interesting would be to understand in what percentage this epigenetic factor affects the onset of the disease. If it were a significant percentage, one could think of drugs then suitable for a large part of the population, otherwise we could talk about personalized medicine. So much remains to be discovered, but somewhere we have to start.
References
[1] Marieke Bax & Tom W. J. Huizinga & René E. M. Toes. The pathogenic potential of autoreactive antibodies in rheumatoid arthritis, Received: 28 October 2013 / Accepted: 1 April 2014 # Springer-Verlag Berlin Heidelberg 2014[2] Qiao Zhong,*,†,‡ Fang-Yuan Gong,* Zheng Gong,* Sheng-Hao Hua,* Ke-Qin Zeng,*,x and Xiao-Ming Gao*,{ IgG Immunocomplexes Sensitize Human Monocytes forInflammatory Hyperactivity via Transcriptomic andEpigenetic Reprogramming in Rheumatoid Arthritis. The Journal of Immunology, 2018, 200: 000–000[3] Daniela Sieghart, Alexander Platzer, […], and Günter Steiner. Determination of Autoantibody Isotypes Increases the Sensitivity of Serodiagnostics in Rheumatoid Arthritis. Front Immunol. 2018; 9: 876. Published online 2018 Apr 24. doi: 10.3389/fimmu.2018.00876[4] Saeed, S., J. Quintin, H. H. Kerstens, N. A. Rao, A. Aghajanirefah, F. Matarese, S. C. Cheng, J. Ratter, K. Berentsen, M. A. van der Ent, et al. 2014. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345: 1251086.

