Stable Relationship between Two Cell
Abstract
The cell communication is important for tissues and organs that remain constant in size and cell composition, and it is mediated by growth factors availability and by extrinsic factors [1]. However, it is not clear whether this can regulate cell homeostasis.
A recent article by Xu Zhou et al. provides an example of how fibroblasts and macrophages communicate to maintain tissue homeostasis [2].
The authors deeply describe the circuit features considering the environmental restriction and negative-feedback regulation. This work is important to further understand cell communicates, interactions and tissue homeostasis.
The ability of organs and tissues to maintain constant size and cell composition, after perturbations such as inflammation [3], is important for the survival of pluricellular organisms. Sustained deviations are often related with pathological conditions for example degenerative and fibrotic diseases.
There are several ways in which homeostasis, and so a stable population of cells, can be ensured. In particular a recent article by Xu Zhou et al. studies the importance of extrinsic factors and the exchange of the growth factor in homeostasis [2]. It is known that cells produce and exchange growth factors and this is important for their proliferation and survival [4].
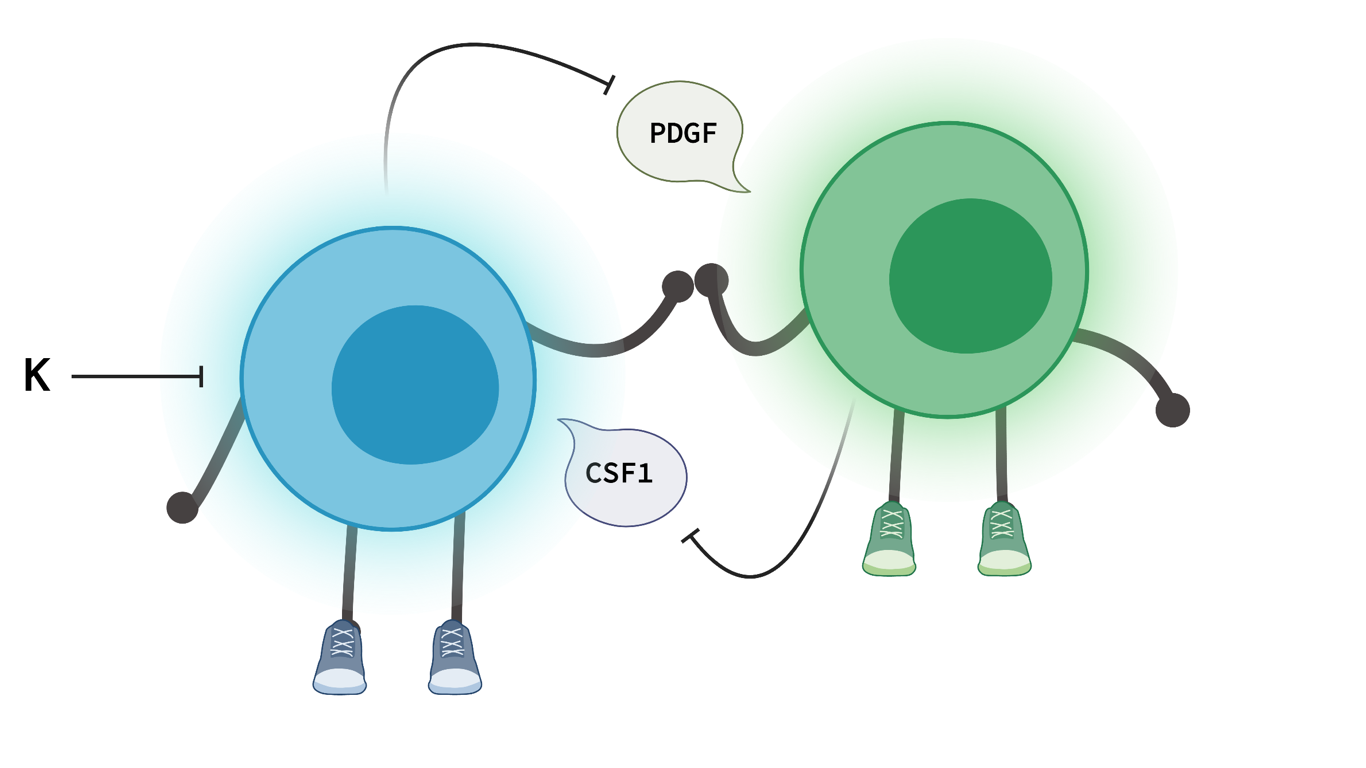
The mutual exchange of the growth factors between two or more cells constitutes a cell circuit with specific properties that guarantee homeostasis [5]. Xu Zhou and collaborators, by employing experimental and computational approaches, have shown that macrophages and fibroblasts constitute a cell circuit through exchange of growth factors [2]. Indeed, using an in vitro system, the authors found that the growth factor Csf1 is highly expressed by fibroblast, while the Csf1 receptor Csf1r) is exclusively expressed on macrophages. Additionally, macrophages express PDGF, and its own receptors, Pdgfra and Pdgfrb, were exclusively expressed on fibroblasts.
In addition, fibroblasts have autocrine loop, composed of PDGF and EGF pathways, that sustains the fibroblasts’ growth. The authors hypothesized that stability of cell composition could be maintained by this cell circuits. The same in silicio screening was used in a study by Camp J. et al. where is investigated the presence of complementary receptor or ligand among pluripotent stem cell (IPSc)-derived human hepatic endoderm (HE), endothelial cells (EC) and mesenchymal cells (MC) [6].Following the kinetics of cell populations, authors found that macrophages and fibroblasts maintain the cell contact during the population growth and reach the constant population ratio independently to the initial ratios. To identify the circuit design features sufficient to maintain this population stability, they used an analytical screening of mathematical models of two cell-circuit.
The cell-circuit showed three states:
- OFF state in which the number of cells is under the critical density so the population tends to zero.
- ON-OFF state when one cell type survival and expansion is supported by an autocrine loop.
- ON state, that leads to the cell homeostasis, in which a population, from a wide range of initial conditions, reaches the stabile ratio.
To favor a stable ON state, it is important that:
- one cell type is limited by carrying capacity, (named K, that is a limitation by extrinsic factors, like space or nutrients)
- the growth factors of the other cells must undergo negative regulation (negative feedback). This model is named spring and ceiling.
The ceiling is the carrying capacity that limits the expansions of one cell type and, interestingly, it is determined by analyzing the proliferation rate. The spring is the negative regulation of growth factors produced by one cell type for the other cell type. Then is demonstrated that fibroblasts are limited by carrying capacity and the autoregulation by receptor internalization plays a major role in growth factors removal.
The authors employed a phase portrait to depict population kinetics and explained how macrophages and fibroblasts proceed from one condition dynamically toward the next. To improve the model, biologically plausible values (necessary to follow the dynamics) have been used to avoid overfitting of the model.
Macrophages and fibroblasts circuit is interesting because it is resilient to perturbations which is important, for example, when inflammatory infiltrate causes changes to cell numbers. It is also proved that fibroblasts’ proliferation depends on the presence of macrophages that express PDGF. In addition, macrophages can not expand in absence of fibroblasts, as well as in presence of Csf1 KO fibroblasts. Therefore, the strong association between macrophages and fibroblasts depended on Csf1.
This study is an important step forward to better understand the tissue stability and organ homeostasis and regeneration. At the same time, it would be interesting to investigate other cell circuit topologies in which other cell can proliferate or too differentiate. Also, there are cell interactions, like in neurons, where the growth factor signal is required just for survival and not for promoting proliferation [7].
It is also necessary to consider that each circuit topology has different characteristics that may change if additional regulatory mechanisms are contemplated. Therefore, it would be important to define which circuits are used by which types of cells.
Recent study showed how cell communication affects differentiation [8]. In particular, Camp and collaborators interrogate signaling in liver buds using a receptor–ligand pairing analysis and a high-throughput inhibitor assay. Those analysis suggest that vascular endothelial growth factor (VEGF) interactions encourage endothelial network formation and hepatoblast differentiation.
Lastly, the authors, hypothesize that each circuit may have specific vulnerabilities to dysregulation. It would be interesting in future studies, to investigate whether the characteristic vulnerabilities of different cell circuits determine specific types of tissue homeostasis disruption, leading to fibrotic, degenerative and neoplastic disorders [8].
References
- Raff, M.C. (1996). Size control: the regulation of cell numbers in animal development. Cell 86, 173-175.
- Xu Zhou et al. (2018). Circuit design features of a stable two-cell system. Cell 172, 744-757.
- Nathan, C. et al. (2010). Nonresolving inflammation. Cell 140, 871–882.
- Hart, Y. et al. (2014). Paradoxical signalling by a secreted molecule leads to homeostasis of cell levels. Cell 158, 1022–1032.
- Hart, Y. et al. (2012). Design principles of cell circuits with paradoxical components. Proc. Natl. Acad. Sci. USA 109, 8346–8351.
- Camp, J. et al. (2017). Multilineage communication regulates human liver bud development from pluripotency. Nature 546, 533–538.
- Crowley, C. et al. (1994). Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell 76, 1001–1011.
- Decker, M. et al. (2017). Leptin-receptor-expressing bone marrow stromal cells are myofibroblasts in primary myelofibrosis. Nat. Cell Biol. 19, 677–688.