The quest for glucose tumor cells vs T cells
Abstract
Establishing why one tumour progresses while others not is a challenge that will last for a long time in the immunology. Nutritional competition between T cells and tumour cells, in particular the glucose, can significantly influence the growth, survival and functionality of both cells. Other factors influencing tumour progression are the presence of proteins and immunosuppressed ligands which can guarantee less attack on tumour cells.
A tumor regression is mediated by effector cells, especially by T cells, responsible for tumor recognition and suppression. T cells use glucose to produce cytokines, while cancer cells use it for its glycolysis, producing lactic acid at the expense of ATP molecules and growing without the need for oxygen.
Chang and collaborators [1] generated a mouse model of tumor regression (R) and progression (P) to investigate this competition. To this end they used a sarcoma cell line D42m1-T2 (tumor R) that expresses a mutant of the rejection antigen, the spectrin-β2. After transplantation, tumor rejection occurs on day 12 by the T cells that produce INF-y. The cell line D42m1-T3 (P tumor) is a progressive clone that lacks this antigen and grows progressively after transplantation.
Based on the theory of nutritional competition, several colculture experiments were carried out. In particular, T cells cocultured with P tumours were more destabilized than those cocultured with R tumors, and produced less IFN-y than T cells cultured in the absence of cancer. Moreover, the amount of IFN-γ produced correlated with the amount of glucose left in the media after culture. When glucose was added to the cocultures the production of IFN-γ increased significantly indicating that the use of glucose is directly correlated with the regulation of the effector function of T cells. It was confirmed that the extracellular acidification rate (ECAR), an indicator of glycolysis, in P tumours was higher than in R tumours, demonstrating that P tumours consumed more glucose.
Also, nutrition has been shown to be critical to tumour progression. R tumour cells were cultured in high glucose and low serum for several weeks, to select R tumour cells with increased glycolysis (R-1%) to compare their growth in vivo with respect to tumour cells akept for the same time in contro media (R). R-1% cancer cells showed improved ECAR and glucose uptake. Tumours R-1% acquired a “progressive “ phenotype although still expressed the mutant β2 spectrin, thus, indicating that the gain of a “progressive” phenotype of R-1% tumours was not due to the loss of a dominant epitope recognized by TIL (Tumour-infiltrating lymphocytes). Furthermore, R and R-1% tumour cells grew at the same rate both in vitro and in RAG – / – mice, which do not have B and T cells; showing that the tumour grows thanks to damage imposed onto the immune response [2].
Next the researchers applied a checkpoint Blockade Therapy. Checkpoints are the key points in the regulation of the immune system processes; they are molecules that act as modulators of the signaling pathways [3].
This therapy activates antitumor immunity by targeting proteins that inhibit T cells, thereby they influence the proliferation of T cells, their function and their absorption of glucose;. As ì the exact mechanisms of how these treatments work remain uncertain, the authors tried to find the reason why they were effective in inducing the regression of progressing tumors and thought that there should be an effect on the metabolism of the tumor and TIL. They injected progressive tumors in mice and treated them with blocking antibodies against CTLA-4, PD1, or PD-L1 and after 12 days assessed metabolic parameters and TIL functions.
From these experiments it has been observed that all blocking antibody treatments led to P tumor regression and that cell glucose levels were higher indicating that blocking antibody therapy corrects the tumor-induced glucose restrictions and restores the glycolytic capacity and effector function of TILs. CTLA-4 is a receptor expressed on T lymphocytes which, following the association with its ligand transmits an inhibitory signal inside the lymphocyte [4]. PD1 is the programmed cell death protein and is expressed on the cell surface with a role in regulating the immune response. PD1 suppresses the activity of T cells to prevent autoimmune diseases , however, it can inhibit the immune system from killing cancerous cells; PD-L1 is its ligand and the link between them induces an inhibitory signal of IL-2 production and T cell proliferation [5].
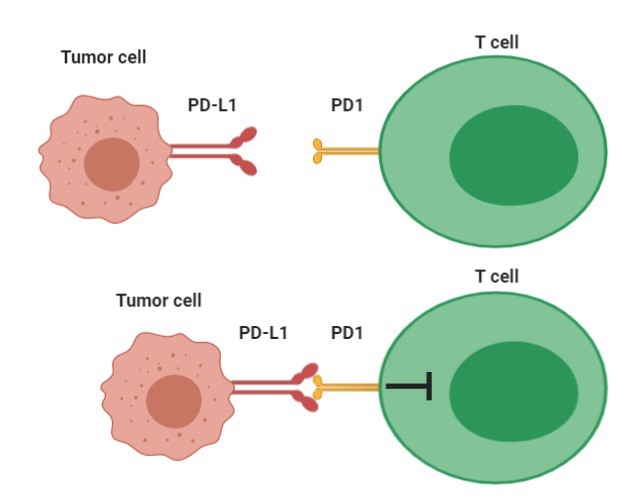
Interaction between PD1 and its PD-L1 ligand induces an inhibitory signal to T cells.
Following treatment with PD-L1 blockers both P and R tumors showed reduced ECAR and glucose uptake, indicating a correlation between PD-L1 and the level of glucose present and absorbed.
They also observed a decrease in the phosphorylation of the target proteins of mTOR and a decrease in the phosphorylation of AKT correlated with the reduction of ECAR; they wanted to determine how the blockade of PD-L1 dampened the mTOR signals in the in vitro system where T cells were absent, in these conditions the relationship between PD1 and PD-L1 could not take place. It was therefore thought that antibodies could cause internalization of PD-L1 and once demonstrated this, they understood that PD-L1 expression on the surface is important for AKT / mTOR signaling in tumors.
These results suggest that PD-L1 is an immunomodulator not only because it sends a negative signal to T cells via PD1 but also because it improves tumor cellular glycolysis and therefore depletes glucose for the immune cellsm Moreover, its expression on the cell surface maintains the AKP / mTOR signaling which in turn supports the translation of enzymes for glycolysis.
In conclusion, we believe that this article has carried out experiments in a fairly exhaustive way regarding the competition between tumor cells and T cells for glucose and above all has shown how PD-L1 blocking antibody therapy leaves more glucose available in the environment tumor cells for immune cells identified a very valid therapy that deserves further studies. It would be interesting to deepen the research based on further nutrients and growth factors and it would also be important to identify the further actions concerning PD-L1 which could also be involved for other nutrients.
References
- Chang CH, Qiu J, O’ Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, W. van der Windt G, Tonc E, Schreiber RD, Pearce EJ, Pearce EL. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Elsevier Inc. 2015;162:1229-1241.
- Birsoy K, Possemato R, Lorbeer FK, Bayraktar EC, Thiru P, Yucel B, Wang T, Chen WW, Clish CB, Sabatini DM. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. 2014;508:108–112.
- Spranger S, Koblish HK, Horton B, Scherle PA, Newton R, Gajewski TF. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. Journal for immunotherapy of cancer. 2014;2:3.
- Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Molecular and cellular biology. 2005;25:9543–9553.
- Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunological reviews. 2010;236:219–242.
- Berghoff AS, Kiesel B, Widhalm G, Rajky O, Ricken G, Wohrer A, Dieckmann K, Filipits M, Brandstetter A, Weller M, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro-oncology. 2014;0:1–12.

