A New Study to Explain Intestinal Stem Cell Regeneration
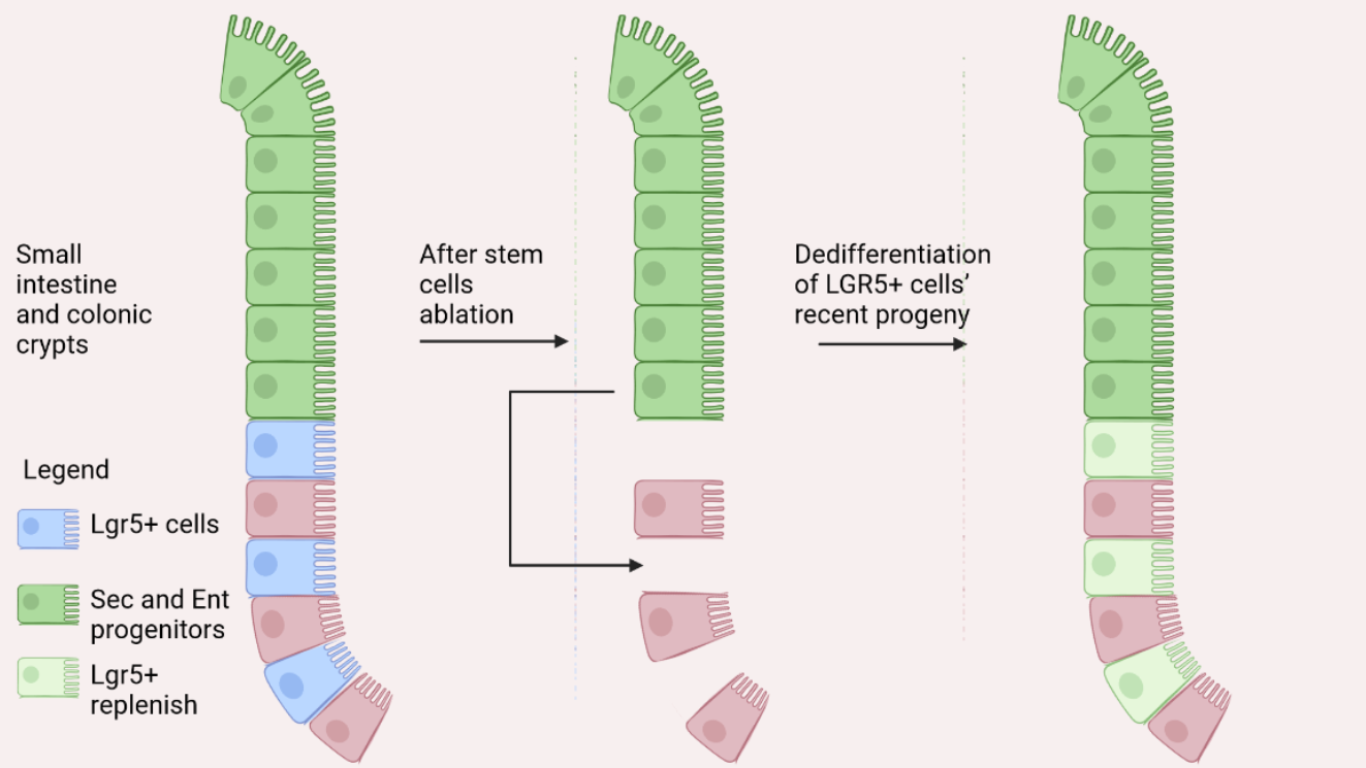
Figure 1 – Injured Lgr5+ ISCs replaced by dedifferentiation of recent stem cell progeny. [Image created with BioRender.com © 2022 all rights reserved]
Asbtract
LGR5+ intestinal stem cells (ISCs), sustain small intestine and colonic epithelial homeostasis. Following injury, the ISCs are soon replenished, but it is unclear if this happens due to activation of a “reserve” pool of quiescent ISCs or by dedifferentiation of recent stem cell progeny. Murata and collaborators [1] show that recovery arises almost exclusively by dedifferentiation of LGR5+ cells’ recent progeny. This work also describes the role of the transcription factor ASCL2 during ISC restoration. This study may be influential to develop novel therapies for intestinal injury.
Discussion
Lgr5+ intestinal stem cells (ISCs) promote colonic and small intestine epithelial turnover. Many studies [2] show that after ISC’s ablation, the tissue recovers thanks to other cells that restore the Lgr5+ cell compartment. A central argument was that the restoration was supported by activation of a “reserve” pool of quiescent ISCs in crypt tier 4. A second hypothesis, however, was based on the dedifferentiation of recent daughter cells. This latter theory is supported by similar epigenetic profile between ISCs and villus enterocyte (Ent) cells [3], and by the observation of specific open chromatin areas in the Secretory (Sec) lineage [4].
To define the mechanisms that underlie regeneration, the Authors [1], used mice as experimental model. When mice were treated with γ-irradiation they selectively lost ISCs. Diphtheria toxin (DT) was also used to ablate ISCs that express the Diphtheria toxin receptor in a specific transgenic mouse model.
Firstly, in a specific mouse model that marks LGR5+ ISCs with a GFP, the Authors irreversibly activated a Tomato (red) fluorescent protein in the ISCs. The Tomato reporter remains active also in the ISC progeny allowing their tracing. These mice were then treated with γ-irradiation and after six days they noticed that restored ISCs expressed progeny’s Tomato reporter. This result was confirmed by flow cytometry of isolated duodenal crypt cells demonstrating that recent stem cell progeny dedifferentiation can replenish the Lgr5+ ISC compartment.
Afterwards, they wanted to identify if each lineage, secretory (Sec) progenitors or enterocyte (Ent) progenitors, can restore ISCs when the other one is depleted. Two different transgenic mouse models were employed: a Sec-cells enriched (Rbpj-/-) intestinal epithelium and an Ent-cells enriched (Atoh1-/-) intestinal epithelium (Fig. 2).
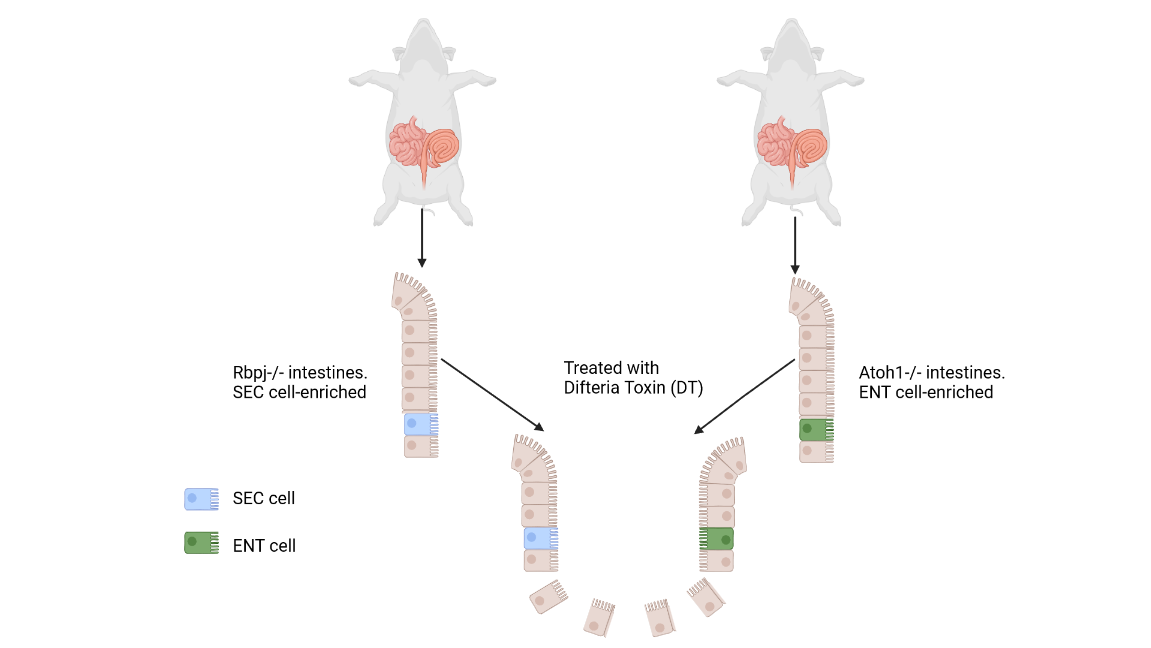
Figure 2 – RbpjFl/Fl or Atoh1Fl/Fl derived mice intestine. [Image created with BioRender.com © 2022 all rights reserved]
Then, after removing the ISCs with the Diphtheria toxin (DT) each intestine was assessed for its ability to restore the ISCs. The analysis revealed that both lineages contribute to the restoration of the ISC compartment, by the dedifferentiation of either Sec or Ent cells.
Previous study [4] noticed that, after ISCs loss, dedifferentiating Sec and Ent precursors extensively alter chromatin accessibility and mRNA expression especially of the Ascl2 locus. For these reasons, the Authors decided to focus their research on the basic-helix-loop-helix transcription factor gene Ascl2. They generated a new Ascl2 allele, the Dana-Farber Cancer Institute allele Ascl2Dfci, to study the role of Ascl2 in the ISCs. It is important to specify that Ascl2Dfci encodes a reporter marker called mCherry (mCh) in frame with the Ascl2 cDNA. The whole transgene can be removed by CRE recombinase leading to Ascl2 allele KO.
Several tests have been performed to analyse the consequences of Ascl2 loss in intestines of healthy mice, which normally expressed Ascl2 in the crypts. After CRE activation, Ascl2 absence did not reveal any signs of malnutrition or disease in the treated mice. Apparently, this transcription factor is dispensable for resting ISC function. However, Ascl2-null crypts are over time replaced by Ascl2-wt crypts, implying that Ascl2 lack in ISCs is disadvantageous over time.
Furthermore, they analysed the effect of Ascl2 depletion in injured crypt cells. In the first case only the Lgr5+ ISCs were ablated, in the second case both Lgr5+-Ascl2+ cells were deleted: only the first mouse survived. In addition, another experiment was performed: Ascl2 KO mice failed to recover in 10 days following DT treatment. These experiments confirm that Ascl2 absence does not seem to have an effect in healthy animals for short periods of time, but its presence is fundamental in animals with damaged intestinal stem cells. Moreover, after ISCs damage, Ascl2 (mCherry) is expressed in both Sec and Ent progenitors, well above the ISC zone. The mCherry+ cells progressively move towards the intestinal crypt base and eventually acquires ISC specific markers. In fact, when these cells were cultured, they were able to generate organoids. Single-cell RNA sequencing (scRNA-seq) analysis also confirms that this mCh+ “upper cell population” expresses ISC-restricted gene markers. So, the Authors can affirm that Ascl2 expression is heavily restricted in Lgr5+ ISCs in homeostasis and during regeneration-associated cell dedifferentiation.
By analysing Ascl2 target genes, a noteworthy candidate has been identified: the IL-11 receptor gene Il11ra1, which has been suggested to play a role in Ascl2-mediated ISCs regeneration.
Conclusions
In summary, this research demonstrates that the intestinal epithelium damage is repaired by the transcription factor gene Ascl2, that drives dedifferentiation of the progenitor cells and restores stemness. From our point of view, this article is very rich in information and experiments to support the results. It would be interesting to investigate if other transcriptional factors, besides Ascl2, play an essential role in the dedifferentiation process. In addition, IL-11 receptor gene Il11ra1 activity and function are very interesting, but further analyses are needed to develop therapeutic IL11-based approaches.
In conclusion, this ground-breaking article could be the forerunner of new studies aimed at resolving ISC-associated diseases as well as provides key mechanisms underlying tissue repair in response to intestinal injury.
References
- Murata K., et al. Ascl2-Dependent Cell Dedifferentiation Drives Regeneration of Ablated Intestinal Stem Cells. Cell stem cell 26, 377-390, March 5, 2020
- Tian H., et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478, 255–259 (2011)
- Kim T.H. et al. Broadly permissive intestinal chromatin underlies lateral inhibition and cell plasticity. Nature 506, 511–515 (2014).
- Jadhav U. et al. Dynamic reorganization of chromatin accessibility signatures during dedifferentiation of secretory precursors into Lgr5+ intestinal stem cells. Cell Stem Cell 21, 65–77.e5, July 6, 2017