Did you know that arsenic influences long-term memory?
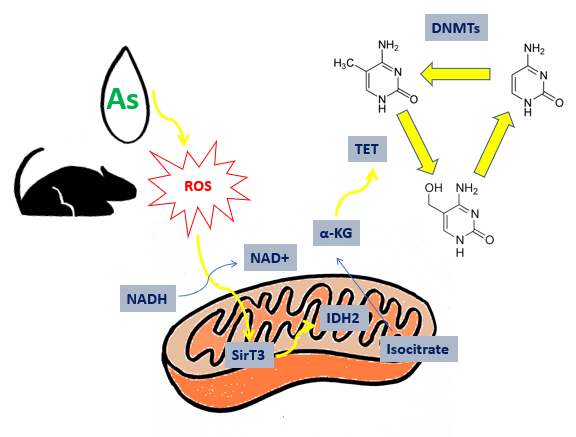
The contamination of groundwater from arsenic induces epigenetic changes that, through the formation of ROS, causes the loss of cognitive and learning abilities, especially in childhood.
Arsenic pollution is a critical problem for the global environment, in particular for those countries that uses polluted water for agricultural productions and human supply. In these areas, mining activity caused by inorganic arsenic (iAs) in the environment, from a concentrations over 50 µg/l. This limit was settled by WHO (World Health Organization) in the ’80s[2]. An example is the case in Bangladesh, where the chronic arsenic exposure caused brain and metabolic dysfunctions. Arsenic-induced disorders cause significant damages to learning abilities and long-term memory. Long-term memory seats in the central nervous system (CNS) within cortex and hippocampus. Their synaptic network is necessary to consolidate information. Damages to neuro-behavioural functions are more relevant in children and adolescents: small doses have a higher impact on young people than adults [3]. These dysfunctions are caused by alteration of epigenetic state of DNA, brought by arsenic-induced oxidative stress. Small doses of arsenic alters the activity of enzyme designated to neutralize ROS (Reacting oxygen species): SOD (superoxide-dismutase) and CAT (catalase) [4]. The epigenetic balance bound to memory is connected to TCA cycle: Sirtuine3 (SirT3) is a nicotine amide dinucleotide (NAD+) dependent protein deacetylase that activates isocitrate dehydrogenase 2 (IDH2). This one catalyses the reaction from isocitrate to alfa-chetoglutarate (α-KG), that modulate the activity of TET enzymes (ten-eleven traslocation). These enzymes have oxygenasic activity and catalyse the formation of 5-hydroxymethylcytosine (5hmC) from 5-metylcytosine (5mC) [5]. The enzyme appointed to formation of 5mC are DNA-methyl-transferase (DNMTs), that utilize S-adenosylmethionine (SAM) like donators of methyl groups. The presence of these bases, different from canonical bases, affects the gene expression: it has been observed that 5mC is involved in gene silencing of CpG islands, as C methylation recruits transcriptional repressors such as MeCP2 (methyl CpG binding protein 2), which completely represses gene expression. The formation of 5hmC is essential to restore gene expression: the transition from methylated to unmethylated form requires necessarily a hydroxymethylated intermediate, which makes demethylation possible.
Then the authors decided to simulate the environmental conditions in laboratory: 30 mice were weaned and divided in three groups then treated with different concentrations of arsenic, chosen according to the doses detected in the highly polluted areas: 7.5 μM, 200 μM and a control group. After six months of treatment, the three groups underwent the Morris test to assess the cognitive abilities influenced by the presence of arsenic. This test showed that treatment does not affect motor skills. However, treated groups had abnormal cognitive and learning abilities compared to the control group. Subsequently, arsenic quantification, performed by mass spectrometry from the cortex and hippocampus tissues, showed a dose-dependent accumulation. The change in gene expression of three categories of genes involved was analysed through RT-PCR: the regulation of neuronal activity, the oxidative equilibrium of the cell, and the Krebs cycle. Additional metabolites (SAM, NAD +, NADH, MDA and α-KG) were quantified by ELISA and quantification assays. The methylation/demethylation status of DNA was then assessed by quantification of 5mC and 5hmC conducted with RP-HPLC coupled to Q-TRAP/MS. The collected data were processed with SPSS statistical analysis software.
The results of RT-PCR on the genes that control neuronal activity show that arsenic induces a down-regulation both in the cortex and in the hippocampus. In particular, a strong decrease in the expression is observed in the cortex tissue in a dose-dependent way of the immediate early genes (IEGs) involved in neural plasticity; instead, calcineurin (CaN) is strongly down-regulated in the hippocampus. The levels of expression of DNMTs and TETs are lower in the treated subjects, especially in the cortex tissue; this induces a lack of regulation of the 5mC and 5hmC levels that leads to the alteration of the normal epigenetic state. The decreasing of the enzymes responsible for the formation of 5mC and 5hmC explains the decline of these two DNA modifications, compromising gene expression. The oxidative stress due to arsenic causes damage at the DNA level: in particular, 5mC undergoes an oxidative deamination process that leads to the formation of T (thymine). This mutation induces a mismatch, with formation of the anomalous couple G (guanine): T. The mismatch repair enzymes are able to recognize the abnormal couple and to correct the wrong base, converting it into unmethylated C. It is clear how to reform a correct pair, compromising the gene expression [6]. SAM levels remain constant in the brain, although they are low in the liver where arsenic is methylated and detoxified [7]. Therefore, the toxicity of arsenic in the brain is caused
exclusively by oxidative stress induced by the metalloid. This hypothesis is confirmed by the results that highlight an over-expression of the genes transcribed during oxidative stress, such as the Keap-1 and Nrf2 transcription factors, involved in the cytoprotective responses, the enzyme heme-oxygenase 1 (HO-1) and the malonildialdehyde metabolite (MDA). Furthermore, oxidative stress causes a decrease in the NADH/NAD+ ratio within the mitochondrion, leading to a lowering of the activity of SirT3 and altering the metabolic pathway of the TCA cycle. Despite this hypothesis, α-KG levels remain almost unaltered, as demonstrated by the results. Therefore, it has not been figured out yet whether there are other pathways that influence the gene expression of the enzymes involved in the regulation and in the epigenetic state of the DNA or if there are brain cells involved in lowering the levels of α-KG, since the analysis were conducted on whole brain and hippocampal tissues.
In conclusion, further studies could be performed on the methylation/demethylation status of the promoters of the genes involved in neuronal plasticity and learning. More accurate analysis could be performed on specific areas of the brain to restrict the types of cells involved in the alteration of cognitive activities and learning.
References
- Xiaoyan Du, Meiping Tian Xiaoxue Wang, Jie Zhang, Qingyu Huang, Liangpo Liu, Heqing Shen, 2018. Cortex and hippocampus DNA epigenetic response to a long-termarsenic exposure via drinking water. Environmental Pollution 234, 590-600.
- L. Smedley, D.G. Kinniburgh, 2002. A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry 17, 517–568.
- Jarup, L., 2003. Hazards of heavy metal contamination. Med. Bull. 68, 167-182.
- Feng, J., Fan, G., 2009. The role of DNA methylation in the central nervous system and neuropsychiatric disorders. Int. Rev. Neurobiol. 89, 67-84.
- Chia, N., Wang, L., Lu, X., Senut, M.C., Brenner, C., Ruden, D.M., 2011. Hypothesis: environmental regulation of 5-hydroxymethylcytosine by oxidative stress. Epigenetics 6, 853-856.
- Feng, J., Zhou, Y., Campbell, S.L., Le, T., Li, E., Sweatt, J.D., et al., 2010. Dnmt1 and dnmt3a are required for the maintenance of DNA methylation and synaptic function in adult forebrain neurons. Nat. Neurosci. 13, 423-430.
- Ríos, R., Santoyo, M.E., Cruz, D., Delgado, J.M., Zarazúa, S., Jimenezcapdeville, M.E., 2012. Methyl group balance in brain and liver: role of choline on increased sadenosyl methionine (sam) demand by chronic arsenic exposure. Toxicol. Lett. 215, 110-118
