G-Quadruplex as targets for Antimalaric Drugs
Malaria is one of the parasites with the highest mortality rate in the world, there were an estimated 219 million cases and 435,000 related deaths in 2017 [1]. Plasmodium is the causative agent of this disease and there are several species: oval, vivax, malariae and falciparum. The latter is the cause of most cases of infection. The transmission vector is a mosquito belonging to the genus Anofelex which carries the parasite in humans through the sting. In the subject the Plasmodium presents an articulated reproductive cycle going to firstly affect the liver and then move to the bloodstream level infecting the red blood cells. Within these, it presents a replicative phase divided into three stages: ring, trophozoite and schizont. In the article by Harris and collaborators [2], the authors analyze the possibility of using specific molecules able to bind the G-quadruplex motifs present in the P. falciparum genome and to block the replicative cycle of the parasite at intra-erythrocytic level. G-quadruplex motifs are secondary structures of DNA and single-stranded RNA. In order to form them, there must be a nucleotide sequence rich in G that complies with the following reason: G3 N1-7 G3 N1-7 G3 N1-7G3. The three-dimensional structure is stabilized by hydrogen bonds between guanines and by electrostatic interactions with monovalent cations such as K +, Li +, Mg ++, Na +.
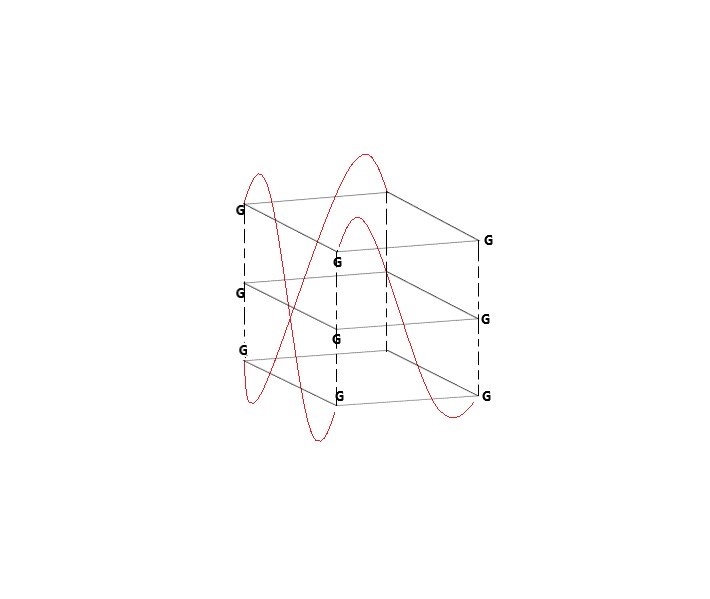
Fig 1. Three-dimensional G-quadruplex structure.
Analyzing the plasmodium falciparum genome, the percentage of G/C present was quantified [3,4]: it is approximately 19% of the total nucleotides. Bioinformatics studies showed the presence of 80 PQSs (G-Quadruplex forming sequences) [5,6] in the non-telomeric regions and 100 PQSs in the telomeric regions. This translates into 1 PQSs approximately every 300 kb. As a first analysis the authors [2] conduct an immunohistochemical assay at the genome level to effectively identify the presence of G-quadruplex motifs in the three stages of intra-erythrocytic development. To conduct this study, they use the IH6 antibody that is specific for G-quadruplexes as demonstrated in mammalian cells [7] results are positive. In the article the four molecules considered, born as anti-cancer drugs, are: Quarfloxin, NMM (N-methyl-mesoporphyrin IX), TMPyP2 (5,10,15,20-tetra-N-methyl-2-pyridyl porphine) and TMPyP4 (5,10,15,20-tetra-N-methyl-4-pyridyl porphine). More attention is paid to Quarfloxin that appears to be able to accumulate naturally in an erythrocytic level where it does not interfere with the metabolism of the infected cell, but acts exclusively on the parasite. In fact, there is not the nucleus in blood’s cell. The EC50 of the four molecules examined is then determined. For this purpose, the SYBR-green fluorescent molecule is used as it is able to bind the DNA of P. falciparum. Following the marking of the genome, they are incubated for 48 hours with the four drugs separately [8]. Assessing the fluorescence loss, the respective EC50 is deduced. To determine whether the efficacy of the molecules is due only to their structure or is also influenced by the affinity for the G-quadruplex are studied the activities of TMPyP4 and TMPyP2, whose structure is very similar, and it is noted that the first has an affinity 18 times higher than the second.
At this point the researchers wanted to measure the “Rate of kill” as previously described [9]. This test uses a genetically modified P. falciparum clone to express a luciferase reporter gene under the control of a specific trophozoite promoter. The suppression of luciferase, caused by the stabilization of G-quadruplexes, causes a loss of bioluminescence. Four compounds whose rate of kill were known (dihydroartemisinin> chloroquine> mefloquine> atovaquone) were used to classify the rate of kill of the four drugs under examination (NMM> quarfloxin> TMPyP2 = TMPyP4). The BRRoK assay effectively measures the rate of kill only in trophozoites.
The cytocidal effects of quarfloxin and NMM were then studied in other stages of the erythrocyte cycle by cytofluorimetric measurement of the DNA content of the parasites. Dead parasites do not synthesize new DNA and do not progress to the next morphological phase. Quarfloxin was highly active against ring stages; and almost equally active against trophozoites, but less active against schizonts. At this point it was clear that these molecules work, however it was not yet clear whether they acted by inducing telomeric malfunctions, inhibiting DNA replication or inhibiting gene expression. A southern blotting experiment was conducted to test whether quarfloxin induces structural changes in telomeres. This test showed that the molecule under examination did not induce abnormal shortening or lengthening in these structures.
To understand if the molecules block the transcription of important genes, genetic engineering was used. For this analysis the genes of the rifin family (PF3D7_0700200 and PF3D7_1254400) were used, which were inserted into a transcription vector with the addition of the fluorescent GFP protein and, subsequently, introduced into the parasites. NMR analysis and thermal resistance study confirmed the effective presence of G-quadruplexes inside the engineered genes. Then the four molecules were tested analyzing the fluorescence loss due to the death of the parasite. From the results it has been possible to deduce that all the four medicines kill the parasite.

Fig. 2
Upper scheme. Gene expression in the absence of stabilized G-quadruplex.
Lower scheme. Inhibition of gene expression in the presence of stabilized G-quadruplex
Finally, the authors investigated the possibility that quarfloxin inhibits the action of RNA Polimerase I by stabilizing the G-quadruplexes of the promoters of genes for rRNAs [10, 11]. Using type A genes (it is transcribed during life in the human host) and a reversal transcriptase PCR they demonstrated that quarfloxin does not affect the activity of the enzyme RNA polymerase I.
In conclusion it can be said that these studies are extremely useful. They allow us to discover new functions of existing medicines and to avoid spending large amounts of time and money on research and experimentation for development of new molecules. It is important to note that these drugs act purely on the parasite, thus, avoiding harmful preventive actions for the ecosystem of which the mosquitoes are part of the parasite. However, the search for a vaccine should still be considered the most important branch of research in this field.
References
- 2018. World malaria report 2018. WHO, Geneva, Switzerland.
- Lynne M. Harris,a Katelyn R. Monsell,a Florian Noulin,a M. Toyin Famodimu,a Nicolas Smargiasso,b Christian Damblon,b Paul Horrocks,a,c Catherine J. Merricka. G-Quadruplex DNA Motifs in the Malaria Parasite Plasmodium falciparum and Their Potential as Novel Antimalarial Drug Targets. American Society for Microbiology 62, e 01828-17
- Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, Paulsen IT, James K, Eisen JA, Rutherford K, Salzberg SL, Craig A, Kyes S, Chan MS, Nene V, Shallom SJ, Suh B, Peterson J, Angiuoli S, Pertea M, Allen J, Selengut J, Haft D, Mather MW, Vaidya AB, Martin DM, Fairlamb AH, Fraunholz MJ, Roos DS, Ralph SA, McFadden GI, Cummings LM, Subramanian GM, Mungall C, Venter JC, Carucci DJ, Hoffman SL, Newbold C, Davis RW, Fraser CM, Barrell B. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498–511.
- Carlton JM, Adams JH, Silva JC, Bidwell SL, Lorenzi H, Caler E, Crabtree J, Angiuoli SV, Merino EF, Amedeo P, Cheng Q, Coulson RM, Crabb BS, Del Portillo HA, Essien K, Feldblyum TV, Fernandez-Becerra C, Gilson PR, Gueye AH, Guo X, Kang’a S, Kooij TW, Korsinczky M, Meyer EV, Nene V, Paulsen I, White O, Ralph SA, Ren Q, Sargeant TJ, Salzberg SL, Stoeckert CJ, Sullivan SA, Yamamoto MM, Hoffman SL, Wortman JR, Gardner MJ, Galinski MR, Barnwell JW, Fraser-Liggett CM. 2008. Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature 455:757–
- Stanton A, Harris LM, Graham G, Merrick CJ. 2016. Recombination events among virulence genes in malaria parasites are associated with G-quadruplex-forming DNA motifs. BMC Genomics.
- Smargiasso N, Gabelica V, Damblon C, Rosu F, De Pauw E, Teulade-Fichou MP, Rowe JA, Claessens A. 2009. Putative DNA G-quadruplex formation within the promoters of Plasmodium falciparum var genes. BMC Genomics 10:362.
- Henderson A, Wu Y, Huang YC, Chavez EA, Platt J, Johnson FB, Brosh RM, Jr, Sen D, Lansdorp PM. 2014. Detection of G-quadruplex DNA in mammalian cells. Nucleic Acids Res 42:860–869.
- Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. 2004. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother 48:1803–1806.
- Ullah I, Sharma R, Biagini GA, Horrocks P. 2017. A validated bioluminescence-based assay for the rapid determination of the initial rate of kill for discovery antimalarials. J Antimicrob Chemother 72:717–
- Kerry LE, Pegg EE, Cameron DP, Budzak J, Poortinga G, Hannan KM, Hannan RD, Rudenko G. 2017. Selective inhibition of RNA polymerase I transcription as a potential approach to treat African trypanosomiasis. PLoS Negl Trop Dis 11:e0005432.
- Drygin D, Siddiqui-Jain A, O’Brien S, Schwaebe M, Lin A, Bliesath J, Ho CB, Proffitt C, Trent K, Whitten JP, Lim JK, Von Hoff D, Anderes K, Rice WG. 2009. Anticancer activity of CX-3543: a direct inhibitor of rRNA biogenesis. Cancer Res 69:7653–7661.