How epigenetic modifications drive programmed genome rearrangement in Tetrahymena thermophila
Abstract
Polycomb group (PcG) proteins and RNA interference (RNAi) are essential in driving the transcriptional repression in many eukaryotic biological processes, especially during development. In this study, Xu and collaborators [1] show that PcG and RNAi have a synergic and fundamental role in histone H3K27 and H3K9 methylation in the ciliated protozoan Tetrahymena thermophila. This mechanism contributes to nuclear differentiation and heterochromatin formation, leading to programmed genome rearrangement.
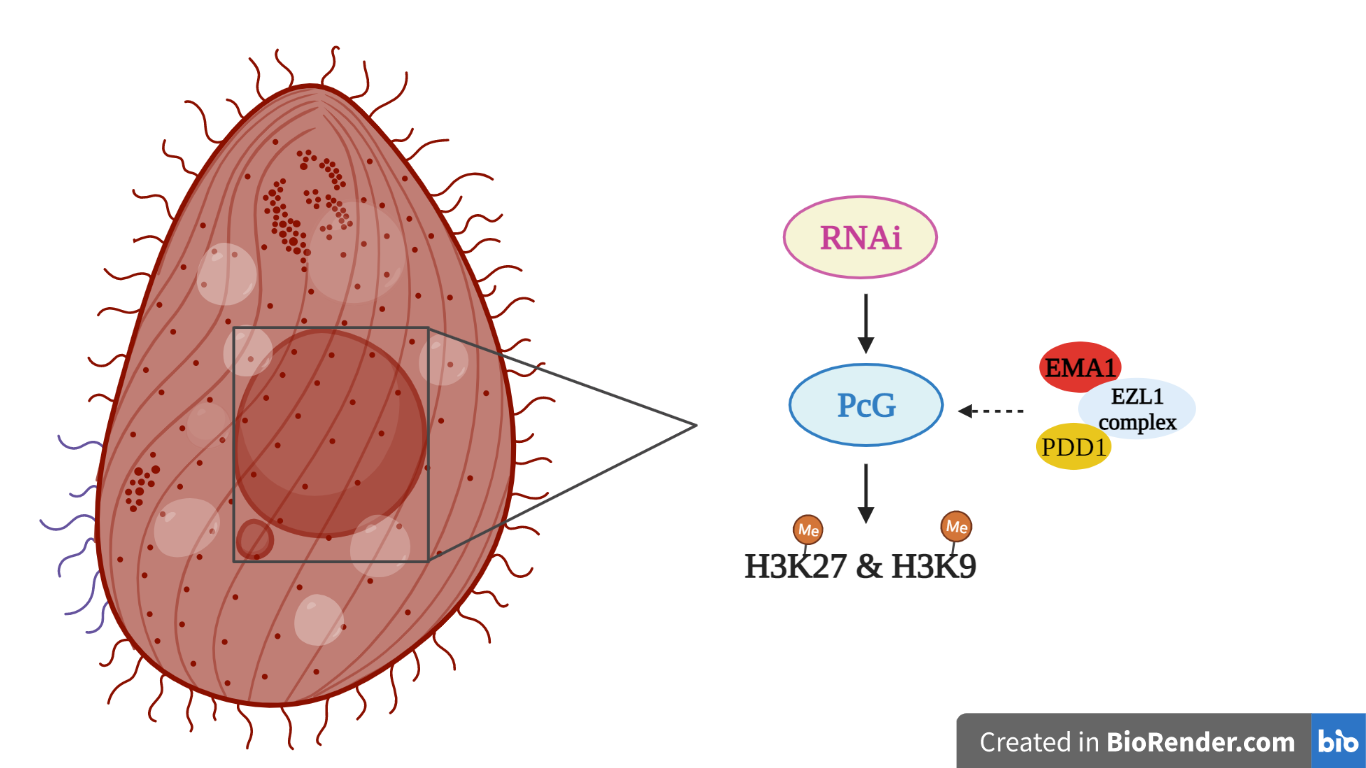
Tetrahymena thermophila is a ciliated protozoan that contains a germinal micronucleus (MIC) and a somatic macronucleus (MAC) in its cytoplasmic compartment. During conjugation, MIC differentiates into MAC. The molecular mechanism starts with RNA Polymerase II (Pol II) that transcripts long non-coding RNA (long ncRNAs) in the MIC [2], which is processed in scanRNA by TWI1 [3]. ScanRNA is paired with nascent transcripts of the developing MAC, and this association is mediated by EMA1 helicase of the RNA interference machinery [2]. The EZL1 complex, an ancestral Polycomb complex, interacts with EMA1 and is recruited to the genomic loci to mediate histone H3K27 and H3K9 methylation. PDD1 protein recognizes methylation and reinforces its association with chromatin. This is accompanied by heterochromatinization and subsequently DNA sequences removal in the developing MAC [4]. In this study, Xu et al. [1] propose that the interaction between PcG and RNAi is involved in transcriptional repression and in dynamic distribution of Polycomb bodies, regulating the programmed genome rearrangement.
First of all, they perform mass spectrometry experiments demonstrating that EZL1 complex is composed by five associated proteins (RNF2, RNF1, NUD1, ESC1, SUZ12), highly conserved in PRC1 and PRC2 of higher eukaryotes. The implication of EZL1 complex in histone H3K27 and H3K9 methylation is shown by immunofluorescence staining, highlighting the overlap between them. By employing functional experiments, they show that the lack of a single component of EZL1 complex abolishes the histone methylation.
The direct interaction between EZL1 complex and EMA1 is demonstrated by experiments of co-immunoprecipitation. Using anti-EMA1 antibodies, EZL1 can be detected. Similarly, using anti-EZL1 antibodies, EMA1 can be noticed too. Furthermore, immunofluorescence staining shows that the co-localization between EZL1 and EMA1 is maintained during all stages of conjugation.
During conjugation of Tetrahymena cells, Polycomb bodies are subjected to dynamic distribution, which includes dispersion and coalescence. An important finding of this work is that dispersion is mediated by nuclear RNAi and histone methylation-binding, that lead to chromatin association, while coalescence is mainly driven by PDD1. The role of nuclear RNAi and histone methylation-binding in the dispersion of Polycomb bodies, is identified by EZL1 complex aggregation in RNAi, EZL1 and PDD1 deficient mutants. On the contrary, the role of PDD1 in coalescence of Polycomb bodies is demonstrated by the dispersion of Polycomb bodies in the loss-of-function mutants of PDD1 and EZL1 complex.
Remarkably, the coalescence of Polycomb bodies leads to programmed genome rearrangement, that occurs through the elimination of the internally eliminated sequences (IES) at the chromosome breakage sequences (CBSs). After removing the repeated DNA IES sequences, the flanking MDS (MAC-destined sequences) are combined by ligation [Figure 1].
Sequencing of the developing MAC genome experiments show that IESs and CBSs are removed in WT organisms (this is demonstrated by the presence of gaps in the sequencing). Importantly, programmed genome rearrangement does not happen in EZL1 and PDD1 mutants.
The role of EZL1 complex in controlling the expression of transposable elements is demonstrated by experiments of RT-PCR, where the expression of transposable elements is measured using WT as control. In WT Tetrahymena cells, transposable elements are not detected, contrary to what happens in mutants. Another transposable element Tc1/mariner mobilizes through a “cut-and-paste” mechanism [5]. The locus that derives from this process is amplified by PCR. Once again, PCR product is detected in mutants and not in WT.
Afterwards, Xu et al. focus on the M element, “an IES retained in the null mutants of the EZL1 complex” [1] and through qPCR they quantify its expression. This analysis confirms that in WT cells, EMA1 and NUD1 are enriched in the M element, unlike most of the mutants. This is also confirmed by RNA immunoprecipitation (RIP) experiments.
Crosslinking immunoprecipitation experiments demonstrate that Pol II interacts with EMA1 and EZL1. Using anti-NUD1 antibody, they demonstrate that NUD1 co-immunoprecipitates with RPB3 and CB20 (Pol II components). In the same way, using anti-EMA1 antibody, there is an interplay between EMA1 and Pol II. A strong interaction between EMA1 and EZL1 is also detected.

Figure 1. Mechanism of programmed genome rearrangement. This mechanism includes histone H3K27 and K9 methylation in the meiotic MIC, the breakage of DNA at methylated sequences during MAC development and the ligation of DNA in the mature MAC.
This study helps us better understand the mechanisms underlying Tetrahymena, but not only. The most important thing is that the programmed genome rearrangement occurs through the elimination of DNA sequences specifically signed by epigenetic marks. In the future, this mechanism could be used as a genome editing technique to act directly on chromatin in the treatment of malformations, genetic diseases and tumors. However, it is a complex mechanism requiring many protein components and many steps that makes the idea challenging.
References
- Xu et al. 2021. A Polycomb repressive complex is required for RNAi-mediated heterochromatin formation and dynamic distribution of nuclear bodies. Nucleic Acids Research doi: 10.1093/nar/gkaa1262
- Aronica et al. 2008. Study of an RNA helicase implicates small RNA-noncoding RNA interactions in programmed DNA elimination in Tetrahymena. Genes & Development. 22, 2228–2241 doi: 10.1101/gad.481908
- Noto et al. 2010. The Tetrahymena argonaute-binding protein Giw1p directs a mature argonaute-siRNA complex to the nucleus. Cell 140, 692–703 doi: 10.1016/j.cell.2010.02.010
- Taverna et al. 2002. Methylation of histone H3 at lysine 9 targets programmed DNA elimination in Tetrahymena. Cell 110, 701–711 doi: 10.1016/S0092-8674(02)00941-8
- Tellier et al. 2015. Mariner and the ITm superfamily of transposons. Microbiology Spectrum doi:10.1128/microbiolspec.MDNA3-0033-2014