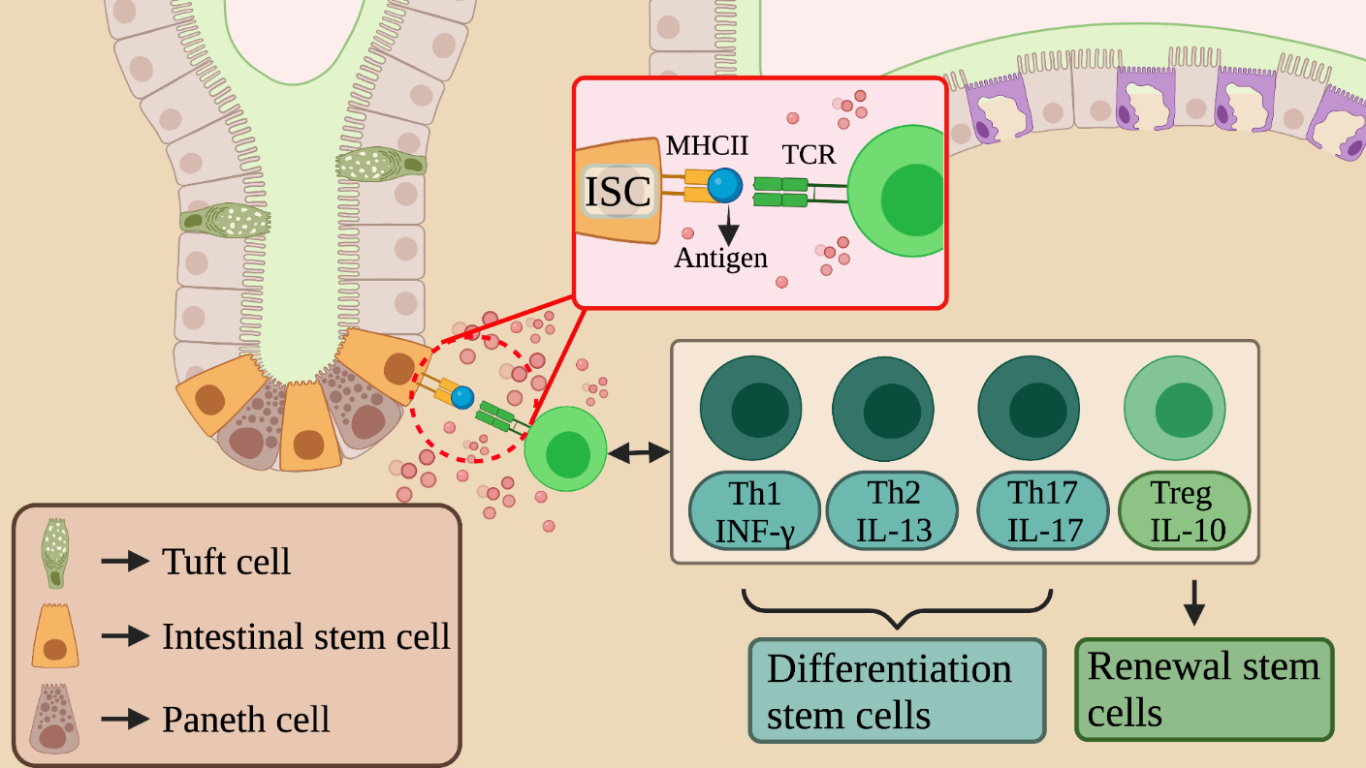
Immune cells drive differentiation of intestinal stem cells
Figure 1 – image shows the possible interactions between ISCs and the immune system cells in the intestinal crypts and highlights how immune cells modulate the differentiation and renewal of intestinal stem cells. Created with BioRender.com
Abstract
In the small intestine, immune cells contribute to the differentiation of the intestinal stem cells (ISCs) to all the epithelial cells. However, the full characterization of this process is largely not understood. In this study, a single cell RNA sequencing (scRNA-seq) is used to characterize the presence of the MHC Class II complex (MHCII) in Lgr5+ ISCs, which makes these stem cells able to present antigens recognized by the T helper (Th). This ISC-Th interaction modulates the renewal and differentiation of the ISCs. The lack of the MHCII complex on ISCs or Th cells has an effect on differentiation also during in vivo infections. This study highlights a new interaction’s axis between gut and immune system that can be instrumental to develop alternative therapeutic approaches against intestinal infections.
In the 2018’ article “T Helper Cell Cytokines Modulated Intestinal Stem Cell Renewal and Differentiation” [1], new axes of interaction between intestinal stem cells (ISCs) and immune system cells were studied, especially focused on T helper and T reg lymphocytes. These cells are able to modulate differentiation and renewal of intestinal stem cells during homeostasis and parasite-mediated infection. The intestinal system is complex and can be divided into different parts having variable proportion of different cell populations. In this study, the variations of stem and other epithelial cells within intestinal crypts were analyzed, in particular after interaction with cells of the immune system. The immune system analyzed in the study belongs to MALT, lymphoid tissue associated with the mucous membranes, and in particular to GALT (gut associated lymphoid tissue), which is found at the intestinal level and is involved in local protection and regulation. An unconventional function of the immune system has been demonstrated by several studies: in particular, the role of Tregs in tissue regeneration processes in muscle and lungs [2], the ability of cytotoxic T cells to deplete Lgr5+ ISCs via MHC Class I complex [3] and the role of skin-resident Tregs in helping to maintain the renewal of hair follicular stem cells (HFSC) through Notch signaling [4]. However, differently from other tissues, a pro-differentiative role of immune cells versus epithelial cells has never been observed in the intestine.
To maintain proper biological function, the intestinal epithelium constantly regenerates, starting from ISCs, which can differentiate into different types of intestinal epithelial cells (IECs). This differentiation is known to be dependent on external signals from both epithelial and non-epithelial cells, such as stromal cells, but a relationship between ISC differentiation and immune cells has never been observed.
In the article, using single-cell RNA sequencing (scRNA-seq), authors found Lgr5+ ISC with enriched expression of MHC class II (MHCII) complex. They showed a new functional axis for gut homeostasis, in which Lgr5+ ISCs can present antigen and interact with Th cells. They characterized the effect of ISC-T cell interactions in organoid assays and in mouse models during homeostasis or infections. They found opposite effects of different Th cells and cytokines on ISC renewal and differentiation.
It is interesting to underline the results obtained under the various conditions of analysis. Following the confirmation of the presence of the MHCII complex on the surface of the ISCs, the effects of the of the MHCII complex interaction with the immune cells were analyzed. The results showed how treatments with IL-13 or IL-17 and co-cultures with Th1, Th2 and Th17 cause a decrease in ISC’s pool; conversely IL-10 and Tregs cause an expansion of stem cells confirming their ability to regulate stem renewal/differentiation.
It is important to highlight that, following Tregs depletion, IECs underwent an aberrant differentiation process, confirming the important role of Tregs on ISCs specification. Further analysis confirmed the necessity of MHCII presence on the ISCs verified by the effects caused after removal of the complex in homeostasis and infection conditions; in both cases this absence causes a deregulation of stem cell differentiation, with a shift of cell populations to stem cells undifferentiated compared to the control analyzed and treated in equal conditions. The ablation of the complex effects also the local immune system, again in both conditions of infection and homeostasis. It has been recorded an increase of dendritic cells compared to the control, suggesting that it may be necessary to compensate the lack of MHCII on ISC.
In conclusion, it is interesting to observe the new axis of interaction found at the intestinal level and the fundamental role that this interaction plays on the modulation of renewal and differentiation of the intestinal stem compartment. However, the role played by ISCs in presenting the antigen to immune system cells via MHCII is an atypical function of conventional stems. Indeed, in order to produce the correct interleukins and stimulation molecules, this function requires the interaction of multiple transmembrane receptors and the formation of an immune synaptic complex [5]. The presence of differentiated cells specialized in this role (dendritic cells) makes surprising the results obtained by this study and raise up questions about the actual capacity of the ISCs in performing this novel function. Further in vivo experiments would be required to fully prove this mechanism.
It would be interesting to further investigate the topic as it’s already known that the immune system has particular axes of interaction, such as the intestine-udder axis [6], which allows to control the whole organism through interactions with unconventional cells. Is the ISC-Th intestine’s axis biologically relevant? Remarkably, the intestine-central nervous system may be relevant [7], since this also involves immune system cells. It is tempting to speculate that alterations in gut microbiota composition could increase the permeability of the gut barrier, therefore activate systemic inflammation and immune responses, regulate the release and efficacy of monoamine neurotransmitters, alter the activity and function of the hypothalamic-pituitary-adrenal (HPA) axis, and finally modify the abundance of brain-derived neurotrophic factor (BDNF), eventually leading to depression condition [8].
In conclusion, it would be interesting to complete the study under Salmonella enterica bacterial infections, in addition to the Heligmosomoides Polygyrus infections analyzed in this work. Therefore, the article not only shows a new essential interaction in regulating ISCs but also suggests the search of new axes of interaction with the intestine.
References
- Biton M, Haber AL, Rogel N, et al. T Helper Cell Cytokines Modulate Intestinal Stem Cell Renewal and Differentiation. Cell. 2018;175(5):1307-1320.e22. doi:10.1016/j.cell.2018.10.008
- Arpaia, N., Green, J.A., Moltedo, B., Arvey, A., Hemmers, S., Yuan, S., Treuting, P.M., and Rudensky, A.Y. (2015). A distinct function of regulatory T cells in tissue protection. Cell 162, 1078–1089.
- Agudo, J., Park, E.S., Rose, S.A., Alibo, E., Sweeney, R., Dhainaut, M., Kobayashi, K.S., Sachidanandam, R., Baccarini, A., Merad, M., et al. (2018). Quiescent tissue stem cells evade immune surveillance. Immunity 48, 271–285.
- Ali, N., Zirak, B., Rodriguez, R.S., Pauli, M.L., Truong, H.A., Lai, K., Ahn, R., Corbin, K., Lowe, M.M., Scharschmidt, T.C., et al. (2017). Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell 169, 1119–1129.
- Santana MA, Esquivel-Guadarrama F. Cell biology of T cell activation and differentiation. Int Rev Cytol. 2006;250:217-274. doi:10.1016/S0074-7696(06)50006-3
- Rodríguez JM, Fernández L, Verhasselt V. The Gut‒Breast Axis: Programming Health for Life. Nutrients. 2021;13(2):606. Published 2021 Feb 12. doi:10.3390/nu13020606
- Martin CR, Osadchiy V, Kalani A, Mayer EA. The Brain-Gut-Microbiome Axis. Cell Mol Gastroenterol Hepatol. 2018;6(2):133-148. Published 2018 Apr 12. doi:10.1016/j.jcmgh.2018.04.003
- Du Y, Gao XR, Peng L, Ge JF. Crosstalk between the microbiota-gut-brain axis and depression. Heliyon. 2020;6(6):e04097. Published 2020 Jun 3. doi:10.1016/j.heliyon.2020.e04097