LDHA in autoinflammatory diseases
Abstract
Aerobic glycolysis is a marker of activated T cells and is involved in the production of the inflammatory cytokine IFN-y through the 3 ‘UTR. the effect of LDHA in increasing the production of the cytokine independently of the 3’UTR, which can create deadly inflammatory damage.
Aerobic glycolysis is a metabolic marker of activated T cells and is involved in increasing the response of these cells, including the production of IFN-y through 3’UTR. In 2016 a group of researchers from Cornell University in New York conducted a study on the function of aerobic glycolysis in the differentiation of T cells, highlighting the role of lactate dehydrogenase in the production of the inflammatory cytokine IFN-y. The study was carried out by comparing the metabolic activity of wild-type mice with that of knockout (KO) cell-specific mice to which the LDHA enzyme was deleted only in T cells.
It was immediately evident that KO cells derived most of their energy not any longer from aerobic glycolysis, but from the Krebs cycle and oxidative phosphorylation, thus following the normal metabolic path of most of the cells of our body. In fact, these cells showed a strong decrease in lactate production, confirmed by a much lower ECAR (extracellular acidification rate) compared to WT cells. In addition, these results have been confirmed by the readings of the Krebs cycle activity, which was enhanced compared to the WT. This thesis strengthened by a very high oxygen consumption rate (OCR).
Furthermore, activation of the glycolytic pathway promoted the expression of IFN-y in activated T cells, but there has been a reduced production of cytokine in KO cells. Considering that the transcription factor T-bet, regulating the differentiation towards TH1, was not influenced, it was hypothesized that the expression of the cytokine was conditioned by other epigenetic mechanisms [3]. First, the 3’UTR region was analysed, both through the link with GAPDH which prevents the translation of the mRNA sequence, and using a reporter, the Yeti allele (yellow-enhanced transcript for IFN-y). Yeti mice were generated by introducing a YFP construct, a fluorescent yellow protein, enhanced by the IRES (Internal Ribosome Entry Site), which led to the translation of IFN-y and YFP from the same mRNA. Bovine growth hormone 3’UTR was also added, used to stabilize the transcription of YFP, thus increasing the sensitivity of the reporter [2]. These studies have shown that in the absence of LDHA, the 3’UTR is not sufficient to mediate the regulation of INF-y.
To evaluate the real reasons that led to the reduced production of IFN-y in T KO cells, the implication of glucose metabolism in the control of gene expression was studied, through an epigenetic mechanism that includes the acetylation of histone H3 on the lysine residue 9 (H3K9ac), a histone marker associated with active transcription [4]. ChIP-seq showed that differentially expressed genes between activated WT and KO cells had smaller quantities of acetylated histone including the Ifng gene. This showed that LDHA promotes the expression of IFN-y regardless of the endogenous 3’UTR, maintaining elevated the concentrations of Acetyl-CoA necessary for the acetylation of the histone which is followed by the transcription of Ifng.
In the absence of the endogenous 3 ‘UTR, the high level of the inflammatory cytokine provoked, in the Yeti WT mice, a lethal autoinflammatory phenotype, associated with serious liver damage and died after a few days. KO mice instead, through the LDHA deletion, corrected liver immunopathology allowing long-term survival [1]. For this reason, LDHA inhibitors are being studied to affect the metabolism of cancer cells and especially for the treatment of autoimmune diseases and drugs against transplant rejection.
In my opinion, this work can represent a good starting point to allow new technologies to progress in the resolution of still incurable diseases, such as tumors and many autoimmune diseases, but it would be interesting to evaluate the possible side effects related to the inhibition of LDHA on the system immune.
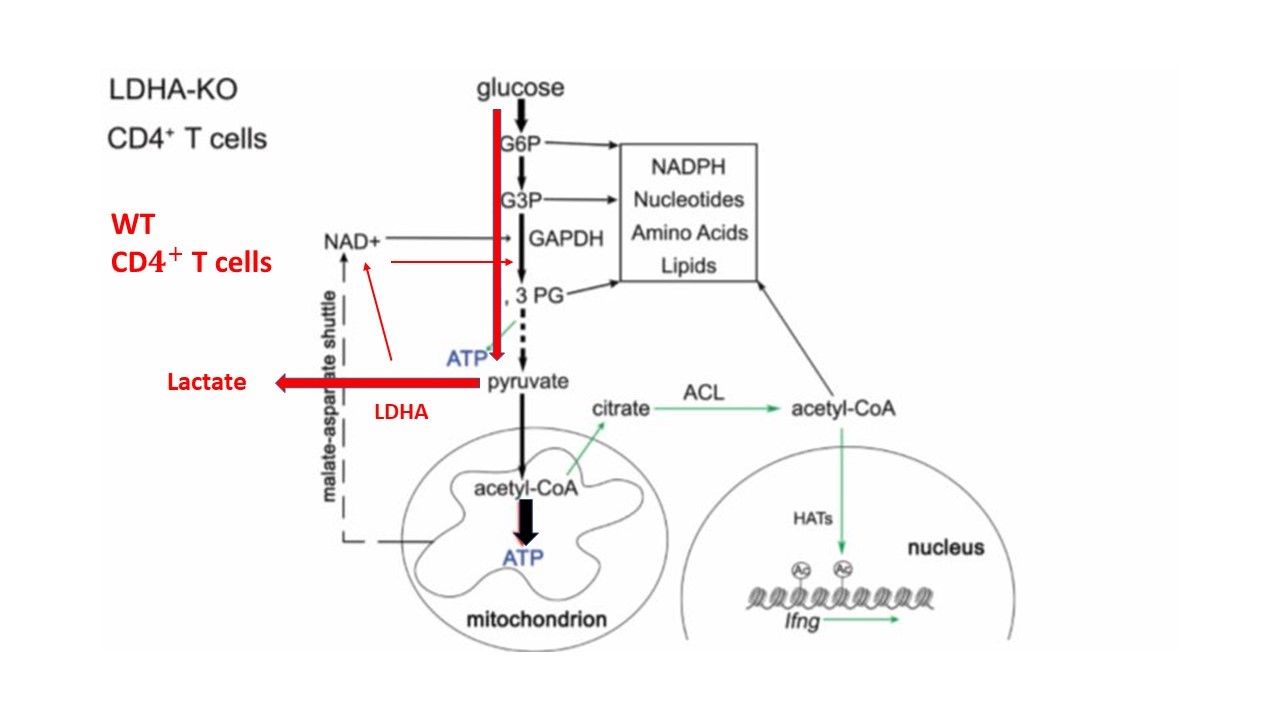
Figure 1: An epigenetic model of aerobic glycolysis in the control of IFN-γ expression
References
- Peng, M., Yin, N., Chhangawala, S., Xu, K., Leslie, C. S., & Li, M. O. (2016). Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science, 354(6311), 481–484.
- Reinhardt, R. L., Liang, H.-E., Bao, K., Price, A. E., Mohrs, M., Kelly, B. L., & Locksley, R. M. (2015). A Novel Model for IFN-γ–Mediated Autoinflammatory Syndromes. The Journal of Immunology, 194(5), 2358–2368.
- Cham, C. M., & Gajewski, T. F. (2005). Glucose Availability Regulates IFN-γ Production and p70S6 Kinase Activation in CD8 + Effector T Cells. The Journal of Immunology, 174(8), 4670–4677.
- Lu, C., & Thompson, C. B. (2012). Metabolic regulation of epigenetics. Cell metabolism, 16(1), 9–17.