The role of SUV39H1 in CD8+ T cell differentiation
A key feature of the vertebrate organisms is to promptly respond to subsequent pathogen exposures thanks to the ability to produce long-lasting T and B lymphocytes during the primary responses. Indeed, once the clonal selection has been completed, T and B lymphocytes can acquire stemness properties (memory cells). Pace et al., in “The epigenetic control of stemness in CD8+ T cell fate commitment “[1] present a deep study of the epigenetic role of the enzyme Suv39h1 on the heterochromatin dynamics in T lymphocytes. This enzyme has a crucial role during the differentiation of T lymphocytes and following the second esposure with the antigen. A more careful understanding of the effects of the enzyme allows for the manipulation of such enzyme in order to develop more effective immunotherapies.
Memory T lymphocytes share with the stem cells the unique ability to differentiate into different cell types, in different tissues. In fact, they are able to self-renew and generate highly specialized tissues [1]. This plasticity is made possible by the contribution of epigenetic factors, which modify the gene expression programs through highly specific chromatin modifications. These epigenetic changes allow a transcriptional control of the cell fate without changing the sequence of DNA, which is the sequence of the nucleotides that make it up. Of note, the acquisition of new and more specific transcriptional programs does not consist only in the activation of certain genes involved in cell differentiation, but also in gene silencing.
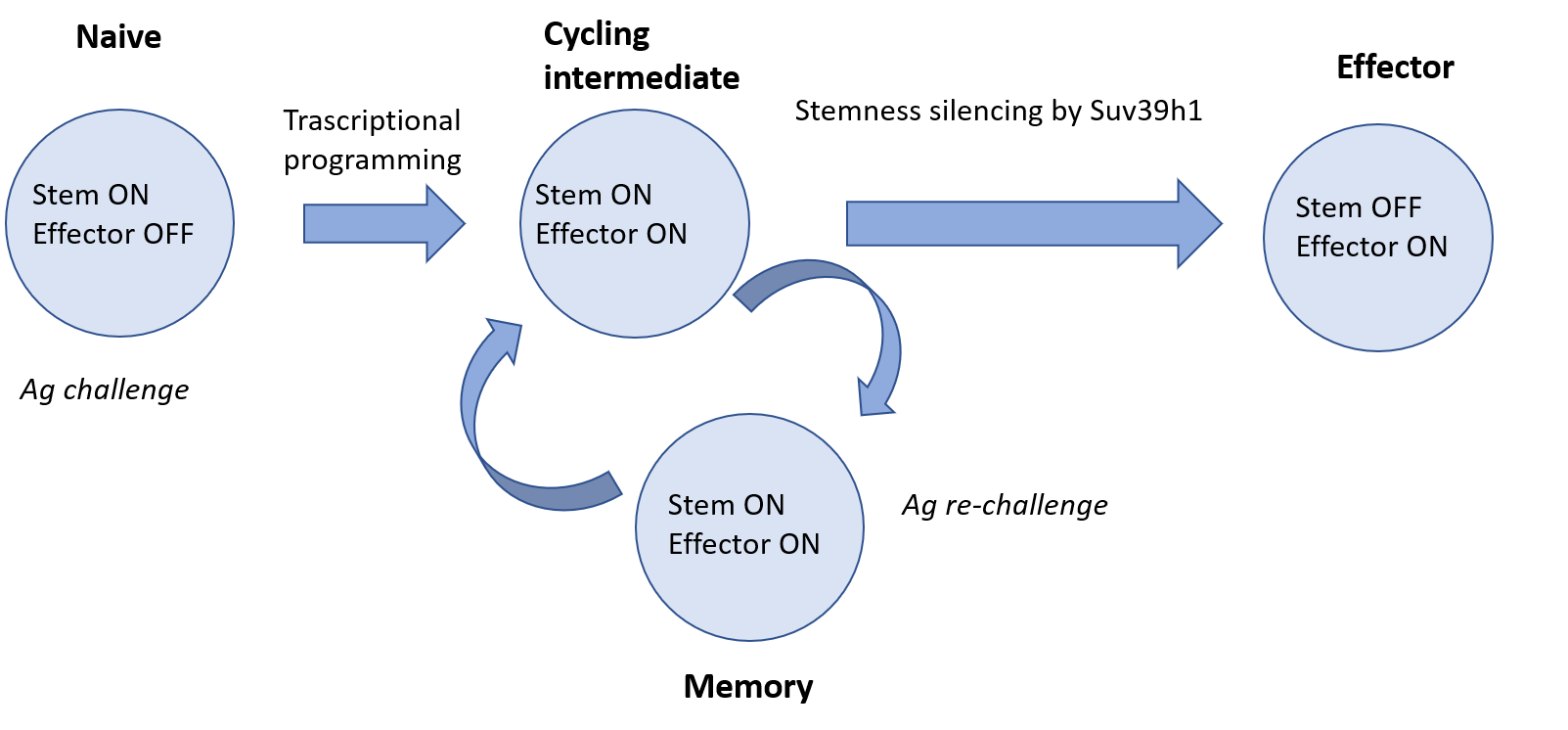
This is the case of Suv39h1, an evolutionarily conserved enzyme involved in the process of constitutive and facultative heterochromatin organization. Suv39h1 is a methyl-transferase which trimethylates lysine in position 9 of histone H3. The work of Pace et al. highlights a new role of Suv39h1 in silencing a set of genes involved in the maintenance of stemness of T CD8+ memory cells, and silenced during the terminal differentiation into T CD8+ effector cells [2]. Naïve T CD8+ lymphocytes, that differentiate in memory cells, following further esposure and before becoming effector, pass through several intermediate levels of growth, which help them becoming T CD8+ memory stem cells, T CD8+ central memory lymphocytes and T CD8+ effector memory lymphocytes [3].
Since Suv39h1 is an epigenetic repressor of the genes associated with stemness, through single-cell RNA sequencing, the authors show that these genes are silenced by Suv39h1 only in the effector subpopulation, whereas these genes are still actively transcribed in CD8+ T effectors deficient for the enzyme. The same analysis highlights a new cycling lymphocyte subset, that is a population of intermediate cells with a double gene expression signature: they are stem memory cells with the ability to leave the cell cycle and get into a quiescence phase, but also cells with effector properties. CD8+ T memory and effector cells have different lifespans: CD8+ T memory cells have a much longer and lasting life, and unique properties of stemness compared to those of the effectors, because of terminal differentiation, these cells quickly go to death. This is the crucial point on which Pace et al has focused on: the activity of Suv39h1 generates a sort of epigenetic barrier, beyond which the cells are committed to become effector and to lose stemness properties, thus failing to differentiating in long-term memory cells. Understanding the control that Suv39h1 exerts on the epigenome is essential for a deeper knowledge of this epigenetic barrier and the mechanisms underlying the differentiation towards the effector pathway rather than towards the way of quiescence. Additional factors could certainly be involved in the process of differentiation. The manipulation of Suv39h1 may be effective in the specific control of certain genes, and, based on the set of potential genes which has been defined, it would be possible to activate and silence them in further experiments, in order to identify which ones are actually involved in the differentiation of memory cells. It would also be interesting to understand the kinetics by which these processes occur, thus succeeding in identifying intermediates, with both stem and effector properties.
The understanding of these epigenetic mechanisms could lead to the development of new therapeutic approaches in the immunotherapy of cancer. T effector lymphocytes, do not possess staminality and therefore plasticity, their epigenetic reprogramming would guarantee a great therapeutic impact on the survival of the cancer patients.
References:
[1] L. Pace, C. Goudot, E. Zueva, P. Gueguen, N. Burgdorf, J. Waterfall, J. Quivy, G. Almouzni, S. Amigorena, “The epigenetic control of stemness in CD8+ T cell fate commitment” Science, 177-186, 2018.[2] A. N. Henning, C. A. Klebanoff, N. P. Restifo, Silencing stemness in T cell differentiation, Science, vol 359, 2018.[3] Abbas-Lichtman-Pober, Immunologia Cellulare e Molecolare, 2002
[2] A. N. Henning, C. A. Klebanoff, N. P. Restifo, Silencing stemness in T cell differentiation, Science, vol 359, 2018.
[3] Abbas-Lichtman-Pober, Immunologia Cellulare e Molecolare, 2002