Transcription factors Atlas as a guide in cell differentiation
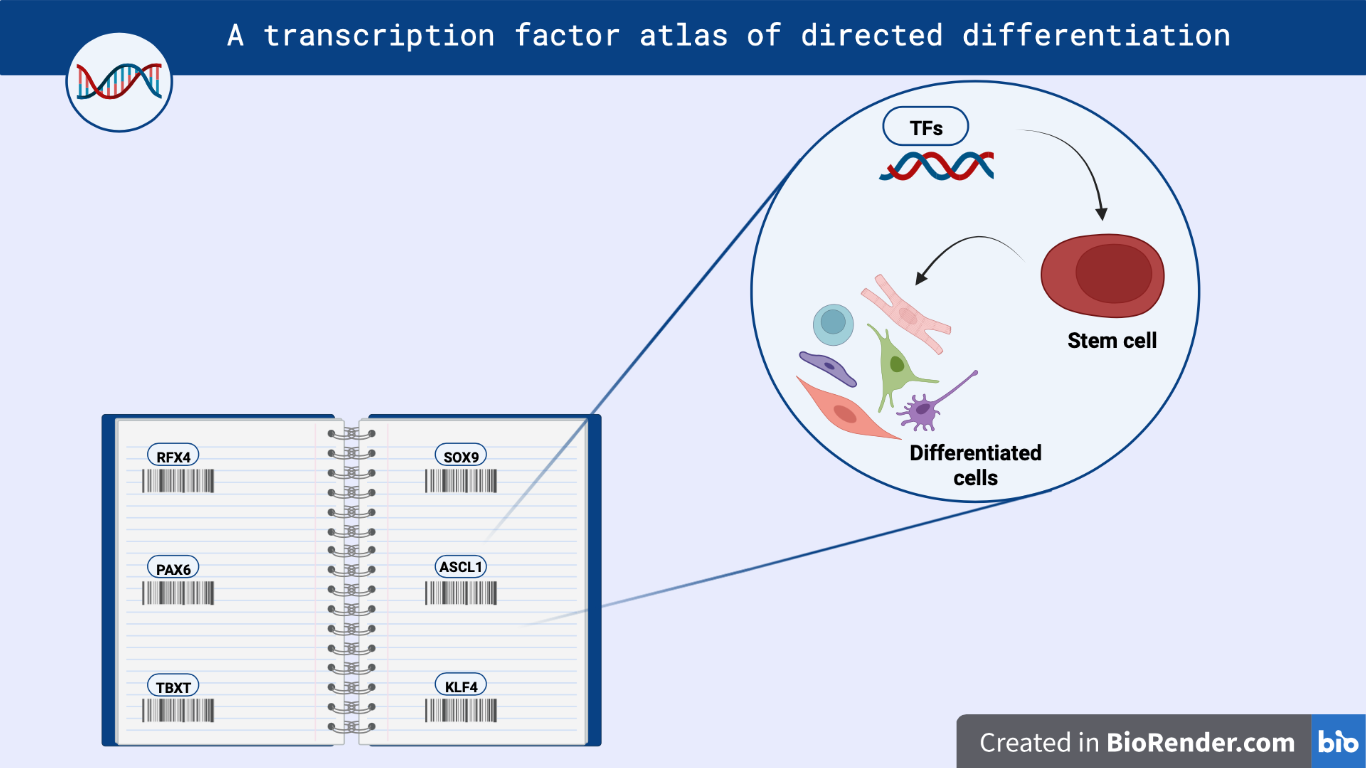
Figure 1 – Image showing the application of TFs Atlas in cell differentiation techniques.
Abstract
Joung et al. have developed a comprehensive transcription factor (TF) atlas that guides the directed differentiation of human pluripotent stem cells (hPSCs) into various cell types. They wanted to understand the function of TFs and the genetic programs they regulate. To achieve this, they created a library of more than 3,500 different splice isoforms of human TFs. The researchers identified specific TFs responsible for directing the development of different cell types and performed targeted screens using subsets of the TF library to create custom cellular disease models. In addition, they examined the effect of combining multiple TFs. Therefore, this study provides a valuable resource that can lay the foundations for a comprehensive understanding of the gene regulatory networks that govern cellular states.
Discussion
Directed differentiation of human pluripotent stem cells (hPSCs) into specific cell types has great potential in regenerative medicine, disease modeling, and drug research [1]. A critical aspect of directed differentiation is the precise control of gene expression, primarily through the action of transcription factors (TFs). TFs are proteins that bind to specific DNA sequences and regulate the transcription of genetic information from DNA to messenger RNA (mRNA), thereby controlling gene expression [2]. Understanding the role of transcription factors in cell differentiation is critical for developing efficient and targeted differentiation protocols. In this work, Joung et al. performed a comprehensive screening to generate an atlas of TFs involved in the regulation of hPSCs directed differentiation.
At first, a comparison of CRISPR activation and open reading frame (ORF) overexpression revealed that ORF overexpression is the most effective approach to increase TF expression [3]. To enable pooled screening, the researchers developed a tool called multiplexed overexpression of regulatory factors (MORF), that is a library of barcoded human TFs in which all isoforms are linked to a unique barcode to allow their unambiguous identification. Human embryonic stem cells (hESCs) were then transduced with MORF library, cultured in STEMdiff APEL medium for 7 days, and finally profiled by single cell RNA sequencing (scRNA-seq).
To investigate how overexpressing TF affects the differentiation of hESCs, they ordered TF-overexpressing cells into pseudotime using two computational techniques, namely diffusion pseudotime [4] and RNA velocity [5], and analyzed the TF expression patterns along the inferred differentiation trajectories. As a result, they found that the expression of genes that drive differentiation (e.g., FBN2, TTN and SOX5) increased over the pseudotime, whereas those that maintain pluripotency (e.g., CD24, LIN28A and OCT4 [POU5F1]) decreased.
Afterwards, the authors mapped the TF-induced expression profiles to those of reference cell types from the human fetal expression atlas [6], obtaining clusters of cells resembling various types from the three germ layers. Each cell cluster contained a unique set of TF-ORFs, suggesting that TF-induced differentiation states are diverse and specific.
To create cellular disease models, the authors selected 90 TF isoforms that are specifically expressed in the induced neural progenitors (iNPs). They transfected hESCs with the selected TFs, allowed them to differentiate and, after 7 days, they tested the presence of iNPs by scRNA-seq and Fluorescence Activated Cell Sorting (FACS), looking at the expression of multiple marker genes simultaneously. They obtained a panel of nine TFs critical for neuronal development. Among those, they focused specifically on RFX4, NFIB, PAX6, ASCL1, which generate multipotent iNPs that can differentiate into neurons and astrocytes.By scRNA-seq, the researchers found diverse induced cell type abundances between the analyzed TFs, with an increased production of central nervous system (CNS) cell types for RFX4-iNPs.
Moreover, they optimized iNPs differentiation by combining the RFX4 overexpression with the SMAD suppression (RFX4-DS-iNPs) [7], obtaining better results as compared to previous iNPs differentiation protocols. They also found that RFX4-DS-iNPs cells were predisposed to differentiate into GABAergic neurons, since the markers NR2F1 and NR2F2 are RFX4 targets.
They subsequently assessed the consequences arising from knocking out of the gene DYRK1A, which is known to be implicated in autism spectrum and overexpressed in Down syndrome. They observed that DYRK1A knock-out increased iNPs cell number, inhibiting neurogenesis. Conversely, in cells with an overexpression of DYRK1A, the proliferation of iNPs was reduced, in agreement with findings from previous investigations.
Ultimately, since TFs often function in combination, the researchers investigated how TF ORFs combine to generate the final expression state. To simulate this phenomenon, they initially generated a scRNA-seq dataset from cells overexpressing 10 TF ORFs and their combinations. Through low-dimensional embedding and cluster analysis, they observed that expression patterns of combinations featuring similar TFs tended to cluster together, occasionally incorporating the individual TF profile of one member within the corresponding pair (CDX1, FLI1, and KLF4).
To validate their predictive methodology, the researchers experimentally examined 12 predicted TF combinations across 3 cell types. The majority of these combinations (11 out of 12) triggered the expression of established marker genes specific to the targeted cell type. Among these, 9 combinations induced higher expression of at least 2 marker genes compared to the individual TF, implying that the TF combination might enhance the faithfulness or efficiency of generating target cell types. This approach promotes the process of cellular engineering by reducing the vast exploration space of combinatorial TF effects, thereby advancing empirical experimentation.
Conclusions
We found this study very interesting as it has high potential in improving cell differentiation protocols and represents an advancement in the field of cell engineering by accelerating efforts to create desired cell types for various applications. However, we believe that for a broader use of this methodology, standard parameters need to be set as, for example, the type of culture media, the time of differentiation and the starting cell type. Nonetheless, the authors have been able to unlock the potential of pluripotent stem cells and pave the way for the development of novel therapies and personalized medicine.
References
- Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126(4), 663-676
- Lambert, S. A., Jolma, A., Campitelli, L. F., Das, P. K., Yin, Y., Albu, M., & Weirauch, M. T. (2018). The human transcription factors. Cell, 172(4), 650-665
- Konermann, S., Brigham, M.D., Trevino, A.E., Joung, J., Abudayyeh, O.O., Barcena, C., Hsu, P.D., Habib, N., Gootenberg, J.S., Nishimasu, H., et al. (2015). Genome-scale transcriptional activation by an engi- neered CRISPR-Cas9 complex. Nature 517, 583–588.
- Haghverdi, L., Bu ̈ ttner, M., Wolf, F.A., Buettner, F., and Theis, F.J. (2016). Diffusion pseudotime robustly reconstructs lineage branching. Nat. Methods 13, 845–848.
- La Manno, G., Soldatov, R., Zeisel, A., Braun, E., Hochgerner, H., Petu- khov, V., Lidschreiber, K., Kastriti, M.E., Lo ̈ nnerberg, P., Furlan, A., et al. (2018). RNA velocity of single cells. Nature 560, 494–498.
- Cao, J., O’Day, D.R., Pliner, H.A., Kingsley, P.D., Deng, M., Daza, R.M., Zager, M.A., Aldinger, K.A., Blecher-Gonen, R., Zhang, F., et al. (2020). A human cell atlas of fetal gene expression. Science 370. eaba7721.
- Schafer, S.T., Paquola, A.C.M., Stern, S., Gosselin, D., Ku, M., Pena, M., Kuret, T.J.M., Liyanage, M., Mansour, A.A., Jaeger, B.N., et al. (2019). Pathological priming causes developmental gene network heterochro- nicity in autistic subject-derived neurons. Nat. Neurosci. 22, 243–255.