A new potential therapeutic target for treating fragile X syndrome
Abstract
Fragile X Syndrome (FXS) is one of the most important genetic cause of cognitive disabilities and autism. The authors analyzed more specifically all the chromatin-associated proteins regulated by Fragile X Mental Retardation Protein (FMRP) and they focused on the possibility of using one of them as a therapeutic target: BRD4.
Fragile X Syndrome (FXS) is one of the most important genetic cause of cognitive disabilities and autism. It affects usually males, approximately 1:4000 males and 1:6000 females [1]. Subjects with this syndrome have both learning and behavioral deficits, for example they can show anxiety, hyperactivity and impulsivity that became very accentuated in adult age [2].
FXS is caused by the loss of Fragile X Mental Retardation Protein (FMRP) due to the DNA methylation of the promoter region of Fmr1 (fragile X mental retardation 1) gene. Specifically it is due to the repetition, more than 200 times, of the CGG triplet in Fmr1 causing the gene silencing [1]. Since FMRP acts as a translational regulator that represses translation of target RNA transcripts, when it is lost there is an over-translation of its target RNAs. Target transcripts of this protein involve synaptic proteins, but also transcriptional regulators called chromatin-associated proteins. Thus, loss of FMRP can result in crucial chromatin changes and misregulation of gene expression.
In their work, published in Cell in September 2017, Korb and collaborators [3] of The Rockefeller University tried to better understand the relationship between FMRP loss and chromatin-associated proteins. Literature has already explained that FXS is involved with alterations of pre- and post-synaptic proteins, and neurotransmitter secretion, however treatments based on these synaptic targets have not been very successful in improving quality of life of patients [3]. They initially focused their study on analyzing FMRP targets by HITS-CLIP technique. They found that a significant number of FMRP targets encoded for chromatin organization and transcriptional factors. Secondly, they performed Western Blot analysis of chromatin-associated targets and pointed out that Brd4, MLL1, p300 are over-expressed in cortical neurons in KO (knockout) condition relative to WT (wildtype). Since these targets regulate histone modifications, loss of FMRP can cause important effects on this level. Moreover, they identified alterations in regions of ‘active’ chromatin such as H3 lysine 4 trimethylation (H3K4me3), H3 lysine 27 acetylation (H3K27ac), and H4 lysine 8 and 16 acetylation (H4K8ac and H4K16ac).
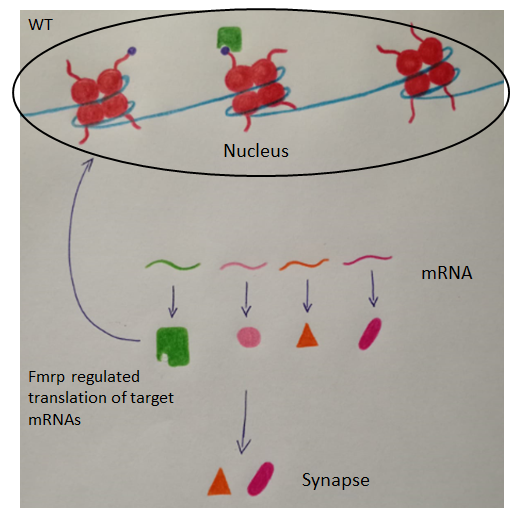
Fig. 1 In WT condition, FMRP regulates translation of mRNAs of both synaptic proteins and chromatin-associated targets, that bind directly chromatin in the nucleus. Among these, there is Brd4 (highlighted in green) which recognizes hyperacetylated regions of chromatin regulating gene expression. FMRP’s loss cause overexpression of proteins including Brd4 and transcriptional misregulation.
Then, these authors wanted to examine which genes were modified the most. Performing chromatin immunoprecipitation and genome–wide sequencing (ChIP–seq), they showed that in KO H3K4me3 was increased Nr4a1 and Shank2 two neuronal genes involved in spine formation. From these data they highlighted that widespread histone modifications are associated with active transcription. Changes in histone modifications can induce a transcriptional misregulation. Considering that, Korn et al. performed RNA-seq to analyze the level of misregulation caused by Nr4a1 and Shank2 overexpression. They showed an increase level of transcripts of genes regulating spine formation. Upregulated genes included also synaptic proteins and receptor proteins. They have also been able to show a significant overlap between genes misregulated in FXS and genes correlated with autism.
In the second part of the study, they analyzed more specifically all the chromatin-associated proteins regulated by FMRP and they focused on the possibility of using one of them as a therapeutic target: Brd4. Brd4 is already known by the scientific community as a crucial cause of cancer, by recognition of hyperacetylated chromatin, it recruits mediator complex promoting transcription of oncogenes [4]. Inhibitor like thienotriazolodiazepine JQ1, is able to compete with Brd4 for hyperacetylated chromatin sites. Blocking Brd4 has been used to treat advanced renal carcinoma [5].
Korb and collaborators used RNA-seq to verify the effect of JQ1 on misregulated genes of KO subjects. First, JQ1 had a very important ability of down regulating most of the genes upregulated, including Brd4, in KO following the FMRP loss. Expanding the analysis to all genes disrupted in KO, JQ1 reversed the expression levels of up regulated genes to almost WT condition, while it didn’t modified the expression levels of down regulated KO genes. Second, JQ1 has been tested for morphological aspects like number of spines. Surprisingly, it was able to reverse the number of spine, regulated by Nr4a1 and Shank2, to wild type levels. Then, they considered the possible effect of the drug on social behavior. They performed the marble burying test in order to measure the levels of anxiety and obsessive-compulsive disorder (OCD) among mice. They used a medium dose of the drug and they saw positive effects on gene expression and on morphological phenotypes. In fact, the treatment of JQ1 was able to reverse the level of anxiety to almost WT. Moreover, authors showed that JQ1 can reverse gene and phenotype alteration of KO subjects, but it can block memory formation in subject with normal level of Brd4 and transcription.
They also overexpressed Brd4 in WT cortical neurons and a phospho-mimic form of Brd4 (acts as phos-Brd4). Both forms recapitulate all the known phenotypes of FXS, specifically phospho-mimic form showed even more an increased alterations. Considering all these data, they advanced the model in which two inhibitors should work together in a synergist way; first JQ1 that inhibits Brd4, second CX-4945 that inhibits kinase CK2. CX-4945 inhibits the enzyme that activates Brd4 by phosphorylating it, increasing alterations.
Certainly, a great innovation of this study is the potential use of a chemical drug as chromatin regulator, that by modifying expression levels of both synaptic and chromatin targets can possibly reverse some of the deficits of FXS. Specifically, it proposes the idea of using drug to act directly on epigenetic modifications, for this reason it opens the possibility of a therapeutic approach for fragile X syndrome. It also highlighted the central role of chromatin regulation in neurodevelopment and gene expression.
Although this impressive work, further studies are needed to applaud these results on human subjects and potentially develop new therapeutic and pharmacologic products.
References
- R. Nick Hernandez et al., Autism Spectrum Disorder in Fragile X Syndrome: A Longitudinal Evaluation, Am J Med Genet A. 2009.
- R. J. Hagerman et al., Fragile X syndrome, Nature Reviews Primer, 2017.
- E. Korb et al., Excess Translation of Epigenetic Regulators Contributes to Fragile X Syndrome and Is Alleviated by Brd4 Inhibition, Cell, 2017.
- B. Donati et al., BRD4 and Cancer: going beyond transcriptional regulation, Molecular Cancer, 2018.
- T. Sakaguchi et al., Bromodomain protein BRD4 inhibitor JQ1 regulates potential prognostic molecules in advanced renal cell carcinoma, Oncotarget, 2018.