CRISPR/Cas9 mediated repair of CFTR in cystic fibrosis patient-derived intestinal organoids
Figure 1 – Schematic illustration of the gene correction protocol. Stem cells are labelled in green. Created with biorender.com
Abstract
Single intestinal stem cells in culture can be expanded to generate organoids exhibiting the architecture of in vivo epithelium. cAMP induces swelling in these organoids by opening the cystic fibrosis transmembrane receptor (CFTR). This effect is not observed in patients with cystic fibrosis (CF). Here it is demonstrated that, through the CRISPR/Cas9 system, CFTR functionality can be restored in cultured intestinal stem cell of CF’s patients [1]. This study reports a valid approach that may be applied in the future to single-gene hereditary defects.
Introduction
It has been previously reported that starting from a single intestinal stem cell (Lgr5+), an organoid can be expanded in culture [2]. This organoid, also called “minigut”, presents all cell types, recapitulating the physiology and architecture of the in vivo epithelium. Furthermore, it has been recently demonstrated that, through the highly efficient CRISPR/Cas9 editing system, it is possible to genetically manipulate these organoids cultures. This system exploits an induced site-specific double-strand break, repaired through nonhomologous end-joining (NHEJ) or, via homologous recombination (HR). In this study, the authors employed CRISPR/Cas9 to perform gene correction in intestinal organoids.
Discussion
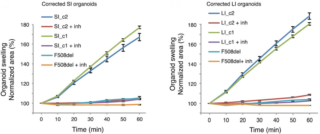
The researchers initially targeted both murine and human APC locus. They used an optimized protocol that includes Wnt culture of intestinal organoids and their transfection with vectors expressing both Cas9 protein and sgRNA targeting APC [3]. Since APC is a negative regulator of the Wnt pathway, stem cells that have both inactivated APC alleles will grow in the absence of Wnt and its R-sponding agonist. In contrast to the growth of wild-type organoids, those carrying the APC mutation have a more cystic appearance. This result holds for both the mouse and human locus. The trial demonstrated the potential of the CRISPR/Cas9 system for gene editing of organoids derived from intestinal stem cells. To apply this system to a precise clinical situation, the researchers considered patients with cystic fibrosis (CF). The effects of this disease on the body are many, including the accumulation of mucus in the respiratory tract and in the ducts (pancreatic and biliary) of the gastrointestinal tract. In particular, they focused on the transmembrane conductor receptor known as CFTR. This receptor codes for an anion channel useful for tissue homeostasis and its mutation is responsible for the disease [4]. They established the small intestine (SI) and large intestine (LI) organoids from two different pediatric CF patients. They considered the most common mutation of the receptor, i.e. the deletion of phenylalanine 508 in exon 11 (F508del), which causes degradation of the CFTR protein [5]. First, they demonstrated the loss of receptor function by performing a test called forskolin-induced swelling assay. Forskolin is a CFTR activator which induces the increase of intracellular cAMP concentration, causing swelling of the organoids. In contrast to the wild-type organoids, no swelling was found in those with the phenylalanine deletion, confirming CFTR loss of function. The researchers then transfected these organoids with a targeting vector expressing the wild-type CFTR sequence and 2 different sgRNAs. The vector also contained a silent mutation and a puromycin cassette system, which were then used to verify the occurred repair, via homologous recombination, of the organoids with the deletion and to distinguish them from those in which it did not occur.
After transfection, the researchers tested the integration of the donor plasmid via PCR. The results showed that the correction occurred on only 16 organoids among the approximately 1000 starting ones. The correction of the F508 deletion was also confirmed by sequencing. It has been previously described that sgRNAs can mis-bind to the target sequence generating off-target indels. To understand this issue, the researchers computationally identified possible off-target sites for the two sgRNAs used. Among the predicted sites, they found, by sequencing, only one site that presented a 4 bp insertion for shRNA1, while no mutations occurred for for sgRNA2. These results show the high efficiency and specificity of the CRISPR/Cas9 system in adult stem cells. They subsequently verified the correction of CFTR function by re-performing the forskolin-induced swelling test. They observed an increase in surface area only in the transfected organoids. Specifically, they observed 177% (±1.4 SEM) and 167% (±3.8 SEM) increase for corrected SI organoid clones and 187% (±3 SEM) and 180% (±1.5 SEM) increase for corrected LI organoid clones (Figure 2). These results were comparable to those obtained for wild-type organoids.
Conclusions
After isolating and expanding intestinal stem cells from two cystic fibrosis patients, the researchers corrected the CFTR F508 deletion using the CRISPR/Cas9 system via homologous recombination and demonstrated the restoration of receptor function in the organoid system. Together with previous studies, this work provides a potential strategy for future gene therapies. However, considering that cystic fibrosis involves multiple organs, the latter does not seem the adequate candidate for a clinical application. However, this approach could in the future be applied to several hereditary defects of a single gene.
References
- Gerald Schwank, Bon-Kyoung Koo, Valentina Sasselli, Johanna F. Dekkers, Inha Heo, Turan Demircan, Nobuo Sasaki, Sander Boymans, Edwin Cuppen, Cornelis K. van der Ent, Edward E.S. Nieuwenhuis, Jeffrey M. Beekman, Hans Clevers,Functional Repair of CFTR by CRISPR/Cas9 in Intestinal Stem Cell Organoids of Cystic Fibrosis Patients, Cell Stem Cell, Volume 13, Issue 6, 2013,Pages 653-658, ISSN 1934-5909, https://doi.org/10.1016/j.stem.2013.11.002.
- Sato, T., Vries, R.G., Snippert, H.J., van de Wetering, M., Barker, N., Stange, D.E., van Es, J.H., Abo, A., Kujala, P., Peters, P.J., and Clevers, H. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesen- chymal niche. Nature 459, 262–265.
- Schwank, G., Andersson-Rolf, A., Koo, B.K., Sasaki, N., and Clevers, H. (2013). Generation of BAC transgenic epithelial organoids. PLoS ONE 8, e76871.
- Cheng, S.H., Gregory, R.J., Marshall, J., Paul, S., Souza, D.W., White, G.A., O’Riordan, C.R., and Smith, A.E. (1990). Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 63, 827–834.
- Dekkers, J.F., Wiegerinck, C.L., de Jonge, H.R., Bronsveld, I., Janssens, H.M., de Winter-de Groot, K.M., Brandsma, A.M., de Jong, N.W., Bijvelds, M.J., Scholte, B.J., et al. (2013). A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat. Med. 19, 939–945.