Drug-screening applications in patient-derived cancer organoids
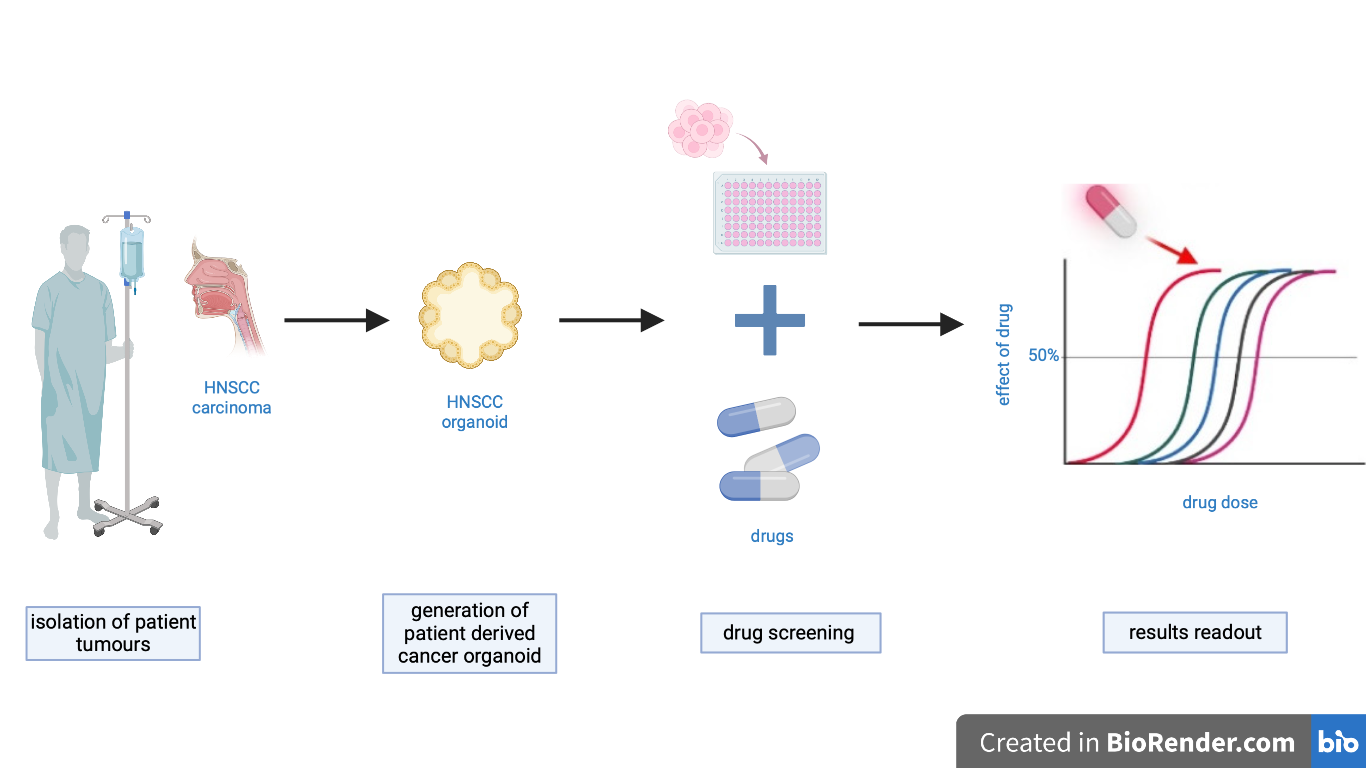
Figure 1 – Schematic representation of establishment of organoids and drug screening, created in biorender.com.
Abstract
In an attempt to move towards a more personalized medicine, research is increasingly embracing drugs screening on 3D cultures e.g. tumor organoids. The generation of cancer organoids from patient-derived material enables a range of therapeutic agents to be tested on 3D cultures. However, cancer organoids establishment and drug screening are still not always applicable. A recent work published in the journal Nature provides a fast, reliable, and efficient protocol for the generation of the head and neck squamous cell carcinoma-derived organoids. Moreover, the authors also develop novel approaches to test in vitro therapy sensitivity of these patient-derived organoids. This study can be applied to other cancers and for other drugs
Discussion
The advent of organoid technology opens new avenues for testing and development of therapeutic approaches in a pre-clinical setting. Generated from various stem cell types and derived from different tissues and even different species, organoids are 3D structures that closely recapitulate tissue architecture and cellular composition. Adult stem cell–derived organoids are generally established from both healthy and pathological tissue and provide an opportunity to expand patient-derived cells in the laboratory [1]. Researchers have begun to investigate whether these cultures are amenable to drug-screening procedures that would allow testing of in vitro sensitivity to various compounds. Patient responses to therapy have been compared to the patient-derived organoid responses. Organoids treatment showed good prognosis indicators, thereby holding great promise for personalized medicine [2]. In this work, Else Driehuis, Kai Kretzschmar, and Hans Clevers have created a general protocol for the establishment of patient-derived organoids and their application for drug screening.
Head and neck squamous cell carcinoma
For the establishment of organoids used in drug screening, researchers generated organoids derived from head and neck squamous cell carcinoma (HNSCC). HNSCC treatment is difficult because of its anatomical location and the highly variability to drug’s response. HNSCCs commonly develop from the mucosal epithelium of the oral cavity with more than half a million patients impacted each year [3].
Establishment of the Protocol
The primary tissue was collected directly from the patients and then transported to the laboratory. The tissue obtained must be checked for the presence of cells different from the epithelial ones because they could limit the organoid growth. Though, if non-epithelial cells are found, they are removed mechanically. Next step involves digestion of fragments into a single-cell suspensions or clumps of cells by using enzymes such as collagenase, DNase, dispase, hyaluronidase or trypsin. After digestion, the cells are plated into drops of an extracellular matrix such as Matrigel to promote cell adhesion and differentiation. Organoids are cultured in growth medium in a humidified incubator at 37 °C. Organoid outgrowth efficiency is dependent on the quality of the sample obtained and the percentage of epithelial tumour cells in the sample. Before drug-screening it is essential to perform a quality control which aims to assess how well the culture recapitulate the patient tumour. So, two different parameters need to be characterized. First, organoids should be representative for the in vivo tumour, and then no contaminant wild type cells should be found. To verify the similarity of the organoids with the in vivo tumour, the bulk DNA sequencing is performed to estimate the frequency of the variant allele as a proxy of the percentage of tumour cells in the culture. If contaminants are found to be present in the culture, then it is necessary to enrich tumour cells in order to decrease wild-type (wt) cells amount. For HNSCC organoids, the factor MDM2 is used to select TP53 mutant tumor cells and inhibit cell-cycle of the wt cells [4].
Drug Screening and Testing
In the screening day, organoids should be subsequently washed and filtered to remove large organoids to reduce variation in the readout of the screening. Lastly, organoids are dispensed into 384-well plates using a ThermoFisher Multidrop dispenser.
The drugs can easily be then dispensed using a drug printer such as an HP D300e digital dispenser. In order to study the several responses, different HNSCC organoids were subjected to gradients of carboplatin agent and Alpelisib, an inhibitor of PIK3CA a tyrosine receptor. To properly assess cell viability during the readout, it is important to include wells containing a compound that kills all cells as a positive control and wells containing the relevant drug solvent as a negative control. These two controls are important to calculate the Z factor that indicate the quality of data generated (Z factor must be > 0.4). The readout is performed at day 5 using CellTiter-Glo 3D Reagent assay which measure intracellular ATP levels, as a surrogate for cell viability. Readout is performed by measuring the luminescence signal from each individual well. Lastly, kill curves (Figure 2a) can be generated to describe the sensitivity of organoids and IC50 values can be extrapolated for quantitative analysis (Figure 2b). IC50 parameter is used to assess the efficacy of a substance and is one of the methods commonly used in drug research to measure the potency of an antagonist. It indicates the concentration of an enzyme inhibitor required to inhibit 50% of the target under investigation [5].
Conclusions
In this study, Dr. Driehuis and collaborators successfully derived the HNSCC organoids from 70% of the plated fragments and provide an efficient method for drug screening in plates (Z factor > 0.7). They have observed a correlation between the in vivo and in vitro response, but more studies are needed to confirm the correlation because the samples used in this research were limited, only 13 to test the effects of increasing concentration of Alpelisib. So, after performing in vitro drug screening a IC50 metric can be obtained for each individual patient-derived organoid and for each therapy. Even if these values indicate in vitro sensitivity, they cannot be directly translated to clinical therapy resistance or sensitivity. In fact, the in vivo tumour microenvironment, patient general health, drug metabolism, patient pre-treatment, and mechanism of action the drug tested may influence either patient response or drug-screening results. For all these reasons, additional studies are required in order to move forward to a precise and successful personalised medicine.
References
- Clevers H. Modelling Development and Disease with Organoids. Cell. 2016 Jun
- Sachs, N. et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell172, 373–386 (2018).
- Johnson, D.E., Burtness, B., Leemans, C.R. et al.Head and neck squamous cell carcinoma. Nat Rev Dis Primers 6, 92 (2020).
- Driehuis, E., Kretzschmar, K. & Clevers, H. Establishment of patient-derived cancer organoids for drug-screening applications. Nat Protoc15, 3380–3409 (2020).
- Sebaugh JL. Guidelines for accurate EC50/IC50 estimation. Pharm Stat. 2011 Mar-Apr;10(2):128-34. doi: 10.1002/pst.426. PMID: 22328315.