Organoids and Tumor Immune Microenvironment: a new strategy of modelling
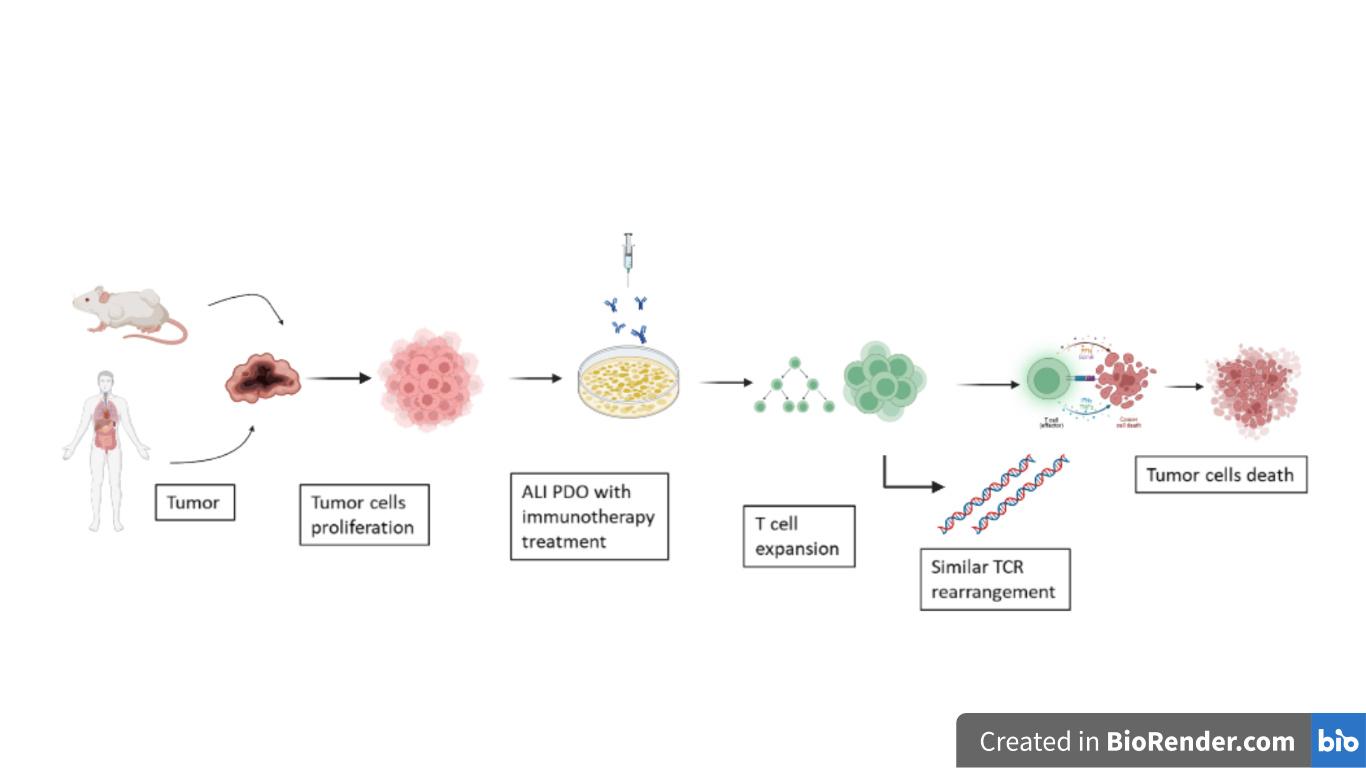
Figure 1 – Schematic representation of ALI PDO establishment starting from human and mouse derived tumor biopsies. All those models faithfully recapitulate the TME and preserve the TCR repertoire. (Created with Biorender.com)
Abstract
Nowadays it’s difficult to produce in vitro cancer cultures which accurately represent the tumor microenvironment (TME). In this article, researchers have tried to create a solution developing a method using an air-liquid interface (ALI) to propagate patient-derived organoids (PDOs) from human biopsies or mouse tumors with embedded immune cells, which can accurately preserve the original tumor T cell receptor (TCR) spectrum [1]. This method successfully modelled immune checkpoint blockade (with anti-PD-1- and/or anti-PD-L1) and elicited tumor cytotoxicity, which could facilitate personalized immunotherapy testing and investigations within the TME.
Discussion
In vitro models to study tumor microenvironment
Today, it is important to choose therapies that involve the manipulation of tumor infiltrated lymphocytes (TILs) to act efficiently in the tumor microenvironment (TME) of human cancer. In vitro models which describe tumor immunity mechanisms do not allow a total analysis of the TME architecture and diversity. Co-culturing of primary epithelial tumor cells with their native immune population remains very challenging. Other reconstitution methods (preservation of murine macrophages, microfluidic devices with human tumor suspensions [2]) did not succeed because immune cells exhibit response to immunotherapeutics, but without tumor-immune specificity [3]. Organoid models offered new promising prospective. First, murine tumor-derived organoids were cultured on an air-liquid interface (ALI) to integrate the epithelium-stroma compartments and to recapitulate tumor environment [4]. Then, the method was extended to patient-derived organoids (PDOs) that faithfully reproduced the histological architecture of the TME, including both the tumor parenchyma and stroma, as well as the TILs [5]. This system allows observation of the immune response within the tumor microenvironment, but not from the peripheral immune system.
PDOs Development and Genetic Characterization
The researchers Neal, Li and Zhu [1] used a methodology to facilitate the study of TME in vitro by preserving the primary tumour epithelium together with the immune and non-immune elements of the stromal component, using PDOs. Initially, they generated organoids in air-liquid interface cultures from murine tissues (intestine, stomach, pancreas). Then, ALI PDOs cultures were also established from 100 tumours of different grades and of 19 different tissue sites. Organoid growth is achieved by using calf serum, but can be accelerated by adding growth factors such as WNT, EFG, NOGGIN to the culture medium [4]. Importantly, PDOs preserved tumor architecture and stroma expressing SMA and vimentin markers.
Using techniques such as targeted-exome sequencing and copy-number variation analysis, genetic alterations were discovered in PDOs, in particular loss of the APC gene in colorectal adenocarcinoma, KRAS mutation in pancreatic duct adenocarcinoma, EGFR mutations in lung cancer, BRAF alterations in thyroid carcinoma [6]. All these mutations observed in PDOs were closely related to mutations observed in the original tumours.
Immune Checkpoint Blockage
Remarkably, ALI-organoid TILs showed activation, expansion and cytotoxicity following treatment with inhibitors for PD1 and PDL1 (e.g. nivolumab) that inactivate the PD1/PDL1 immune checkpoint. Indeed, researchers evaluated the immune response over a 7-day period by analyzing the transcripts of genes involved in the immune response (IFNG, GZMB, PRF1) [7].
The method used not only preserved T cells, but also macrophages, B cells and NK cells. In general, both organoid models show a progressive decrease in immune stroma and fibroblasts in about 1-2 months; however, the loss of TILs can be delayed by using IL-2 or anti-CD3/anti-CD28.
PDOs Recapitulate TCR and Ig Repertoire of Original Tumor
The use of Chromium Immune Profiling allowed a remarkable amplification of alpha and beta chains of TCRs or Ig heavy/light chains considering single cells. This technique, which simultaneously identifies the transcriptome and the various TCR/Ig rearrangements at single cell level, allowed the researchers to effectively discriminate T and B cells clonality. Using single-cell transcriptome assay it was shown that the PDOs of the various donors preserve the main immune repertoire. Furthermore, by means of the VDJ enrichment analysis it was deduced that the clonotypes of the most highly expressed TCRs in biopsies of original tumors are extremely well represented in the respective organoids.
As last step, a single-cell FACS-based method confirmed that organoids accurately recapitulate the repertoire of dominant TCRs present in original tumours, but the number of TILs is lower.
Conclusions
In conclusion, these PDO models can be used to test immunotherapies against cancer, but further optimizations are required aimed to define the contribution of the peripheral immune system. However, PDOs allows the identification of the immune response localized within the TME.
This work also studies the use of anti PD1 and PDL1, and they found that generates blockade of the PD1 axis within the TME and activates and expands TILs. However, the effectiveness of this immunotherapy mechanism is still limited, as only a small fraction of patients responds to PD1/PDL1 targeting.
PDOs could also be employed to study other immunotherapies by targeting other immune cells and their receptors. However, future studies are needed to establish definitive correlations between organoids and patient immunotherapeutic responses. One future prospective is the achievement of new approaches to culture PDOs incorporating other immune and stromal components to develop more precise cancer therapies.
References
- Neal et al., (2018). Organoid Modeling of the Tumor Immune Microenvironment. Cell 175, 1972–1988
- Chen, J., Lau, B.T., Andor, N., Grimes, S.M., Handy, C., Wood-Bouwens, C., and Ji, H.P. (2018). Single-cell transcriptome analysis identifies distinct cell types and intercellular niche signaling in a primary gastric organoid model. Bio Rxiv.
- Deng, J., Wang, E.S., Jenkins, R.W., Li, S., Dries, R., Yates, K., Chhabra, S., Huang, W., Liu, H., Aref, A.R., et al. (2018). CDK4/6 inhibition augments anti-tumor immunity by enhancing T-cell activation.Cancer Discov. 8, 216-233.
- Ootani, A., Li, X., Sangiorgi, E., Ho, Q.T., Ueno, H., Toda, S., Sugihara, H., Fujimoto, K., Weissman, I.L., Capecchi, M.R., and Kuo, C.J. (2009). Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 15, 701–706.
- Neal, J.T., and Kuo, C.J. (2016). Organoids as models for neoplastic transformation. Annu. Rev. Pathol. 11, 199–220.
- Bailey, P., Chang, D.K., Nones, K., Johns, A.L., Patch, A.M., Gingras, M.C., Miller, D.K., Christ, A.N., Bruxner, T.J., Quinn, M.C., et al.; Australian Pancreatic Cancer Genome Initiative (2016). Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531, 47–52.
- Herbst, R.S., Soria, J.C., Kowanetz, M., Fine, G.D., Hamid, O., Gordon, M.S., Sosman, J.A., McDermott, D.F., Powderly, J.D., Gettinger, S.N., et al. (2014). Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567.