Transplantation of intestinal organoids into murine models
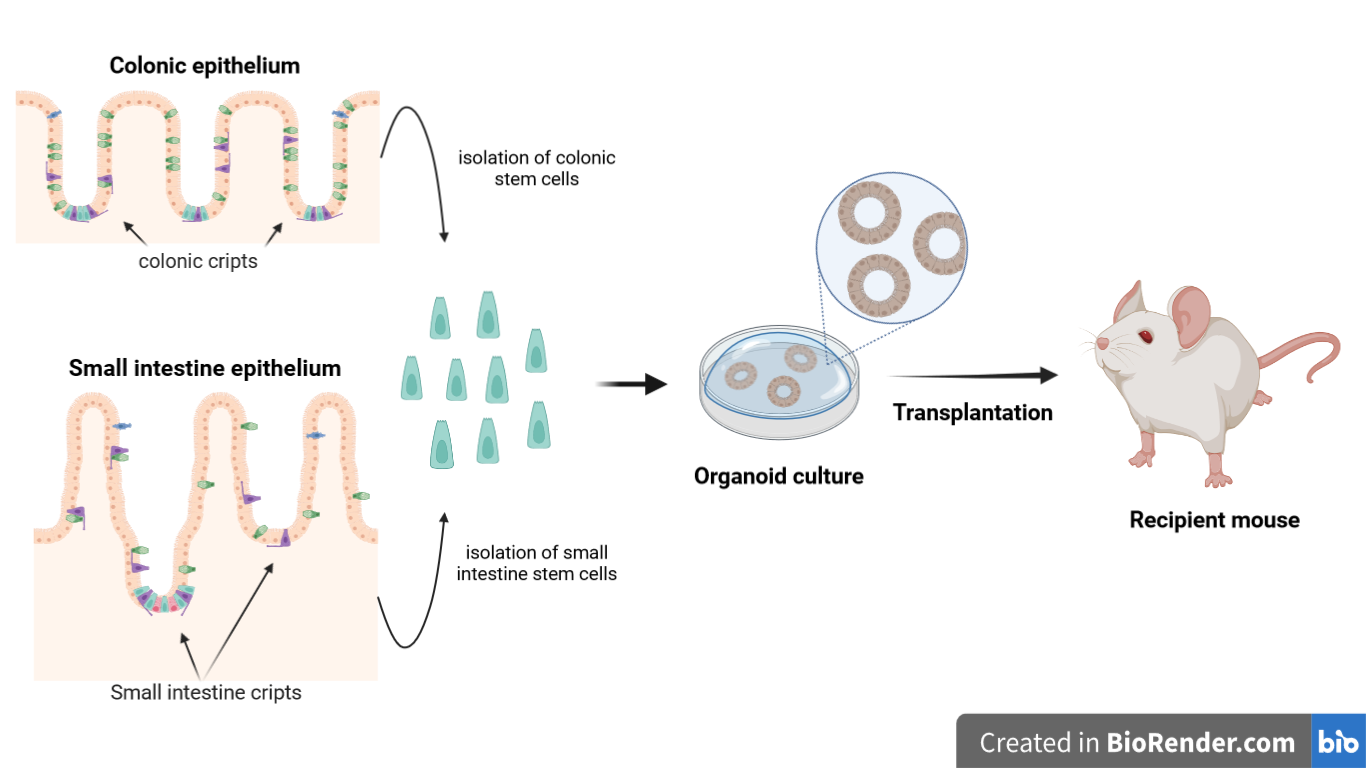
Figure 1. Schematic illustration of procedure through which the transplantation of intestinal organoids is performed
Abstract
Intestinal organoids are in vitro tools that can be transplanted in vivo. They can also be used for disease modelling, in regenerative medicine and to study stem cells potential. Here we describe in detail how to orthotopically transplant epithelial organoids into the colon of recipient mice [1]. In this assay, epithelial injury is initiated at the distal part of colon by the administration of dextran sulfate sodium (DSS), and organoids are infused into the luminal space. The infused organoids attach to the injured region and regenerate a donor-derived epithelium. The assay has been applied successfully to organoids derived from genetically modified cells of colonic and small intestinal (SI) epithelium. This is a versatile protocol for transplantation following colonic injury models. It can been used to form the basis for the first in-human clinical trial using colonic organoid transplantation therapy for intractable cases of ulcerative colitis.
Introduction
The intestinal epithelium is important for nutrient uptake and for defence against the luminal environment of the intestine [2]. In case of inflammatory bowel disease, the intestinal epithelium is subjected to damage. In the circumstance in which the damage is so extensive, traditional cure is not effective. One potential future approach would be to resort to use intestinal organoids to repair the damaged small intestine (SI) or colon. Organoids are 3D cell cultures generated from adult stem cells isolated from intestinal crypts [3,4]. These organoids are transplanted into recipient organisms in order to treat inflammatory bowel diseases, including ulcerative colitis [5,6]. Here it will be explained how to perform an orthotopic transplantation of these organoids in murine models.
Animal Cohort
To carry out the transplantation, it is necessary to follow a protocol that employs Rosa26mT/mG as donor mice and RAG2-I- or C57BL6J as recipient mice. Rosa26mT/mG is genetically modified to express the fluorescent protein TdTomato. C57BL6J has the same congenital background as the donor, while RAG2-I- is used because it is immunosuppressed.
Discussion
Injury model
Recipient mice are exposed to DSS in order to induce epithelial damage in the distal region of the colon. This damage is essential for the infusion and the integration of organoids and the subsequent regeneration of the damaged epithelium. DSS is administered through drinking water for about 7 days after which the mice are weighed: if the cohorts have suffered a loss of 10% of the initial weight, then the epithelial damage has occurred successfully. The experiment can then proceed with the transplantation of the organoids.
Preparation of enema and infusion of organoids
The next stage of the assay involves preparing an enema containing a solution that includes organoids. The recipient mice are then subjected to anesthesia using a 4% isoflurane mixture. Now, organoids transplantation happens by inserting the enema inside the anus of recipient mice with subsequent application of istoacrylic glue to avoid loss of the solution.
Tissue isolation
After about 3 weeks, the process of integrating organoids into the damaged epithelium and its regeneration ends. Tissues can be isolated and subsequent microscopy analysis performed in order to identify the area of the graft. In the tissue isolation the colon needs to be dissected from anal verge and it is followed by consecutive washes. Finally, a longitudinal section of the affected segment of colon is performed.
Detection of engraftment
The portion of the colon is subjected to analysis under the stereomicroscope: a type of microscope suitable for the analysis of freshly isolated samples. The grafted area is identified by the fluorescence signal induced by the integration of organoids expressing TdTomato. The sections tested positive for grafting are fixed with paraffin, placed in a solution containing sucrose together with PBS and subjected to cryopreservation. These are then subjected to the action of the cryotome which allows the formation of consecutive sections of the graft. The latter are exposed to fluorescently labeled antibodies directed against specific markers to proceed with histological analyses.
Conclusions
Histological analysis revealed that intestinal organoids integrate into the damaged areas. The comparison of the engraftments between cohorts of RAG2-I- and C57BL6J, showed that a transplant performed on RAG2-I- has a higher probability of success than one performed on C57BL6J. Measuring the TdTomato-expressing areas, the researchers showed that engrafted areas generated from colon organoids are bigger then those generated from SI organoids in both the two types of recipient mice. In second place engrafted areas generated by transplantation of organoids are bigger in RAG2-I- then in C57BL6J: this result is confirmed in both the colon and SI organoids.
In further histological analysis specific markers for epithelial, colon and SI cells are used. Thus, it is concluded that a new layer of epithelial cells is generated following organoid transplantation. Furthermore, colonic tissue is generated by transplanting colonic organoids. In contrast, transplanting SI organoids generated SI epithelium.
Limitations
Although this protocol is reproducible and simple to implement, there are some limitations. Firstly, cells are infused into the lumen of the colon without prior or subsequent visualization using colonoscopy. Secondly, the model relies on the induction of colitis. It is not sure that the protocol will have the same results even if the epithelial damage is induced by an inflammatory bowel disease. Moreover, only two strains of recipient mice are used in the assay: it is not certain that DSS exerts the same effects on other recipient strains. Thirdly, epithelial injury is primarily localized in the distal part of colon, and any engraftment is consequently only found in this part of the intestine.
References
- Watanabe, S. et al., Transplantation of intestinal organoids into a mouse model of colitis. Nature 17,649-671 (2022).
- Van der Flier, L. G. & Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Rev. Physiol. 71, 241–260 (2009).
- Yui, S. et al., YAP/TAZ-Dependent Reprogramming of Colonic Epithelium Links ECM Remodelling to Tissue Regeneration. Cell Stem Cell 22, 35–49e37 (2018).
- Sato, T. et al., Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
- Yui, S. et al., Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Med. 18, 618–623 (2012).
- Fordham, R. P. et al., Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell 13, 734–744 (2013).