Defining Distinct Colorectal Cancer Models: New Therapeutic Perspectives
Figure 1 – Investigation of molecular differences and immune microenvironment in MSS and MSI-H CRCs performing in silico, in vitro and in vivo experiments.
Abstract
In the gastrointestinal (GI) tract, specifically in the colon-rectum region, different cell populations can be identified based on their position in the intestinal epithelium. Stem cells can be found at the base of the crypts: they are responsible for cell renewal and regeneration. Differentiated cells, on the other hand, are mostly located near the epithelial surface. Colorectal Cancers (CRCs) show different characteristics based on their cellular origin and can be subdivided into Microsatellite Stable (MSS) CRCs and Microsatellite Instability-High (MSI-H) CRCs. In this study, the Authors, using a multi-omic approach, tried to define the molecular differences between the two tumor subtypes. This study highlights the existence of two distinct tumorigenic processes. This information can be useful for creating personalized therapies.
Discussion
Colorectal Cancers are one of the leading causes of morbidity and mortality worldwide [1]. According to the Fearon and Vogelstein model [2], CRC is the result of a progressive accumulation of mutations of specific genes: the most common lead to the hyperactivation of the WNT-pathway, to the inactivation of oncosuppressor genes (e.g. TP53) and/or activation of oncogenes (e.g. KRAS). However, more recent studies revealed two types of CRCs (Figure 2): Microsatellite Stable CRCs and Microsatellite Instability-High CRCs that can develop, respectively, from pre-cancerous conventional adenomas (ADs) and serrated polyps (SERs). ADs are usually located at the crypt base, whilst the SERs are located at the luminal surface.
In the article “Differential Pre-malignant Programs and Microenvironment Chart Distinct Paths to Malignancy in Human Colorectal Polyps” published on Cell in 2021 [3], the Authors, using a multi-omics approach, tried to categorize and define what are the characteristics of human colorectal polyp subtypes. The aim of the paper was to create a single-cell transcriptomic and imaging atlas to clarify the molecular mechanisms involved in the transition from a pre-cancerous state to cancer.
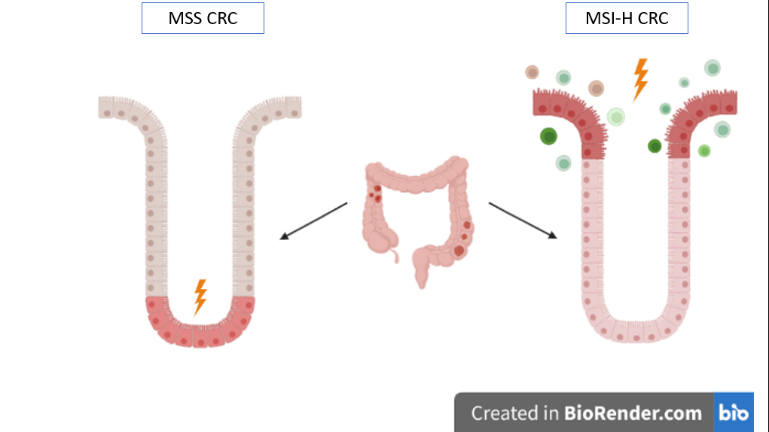
Figure 2 – Position of MSS CRCs and MSI-H CRCs.
The ADs and the SERs can be histologically categorized into subtypes (Figure 3).
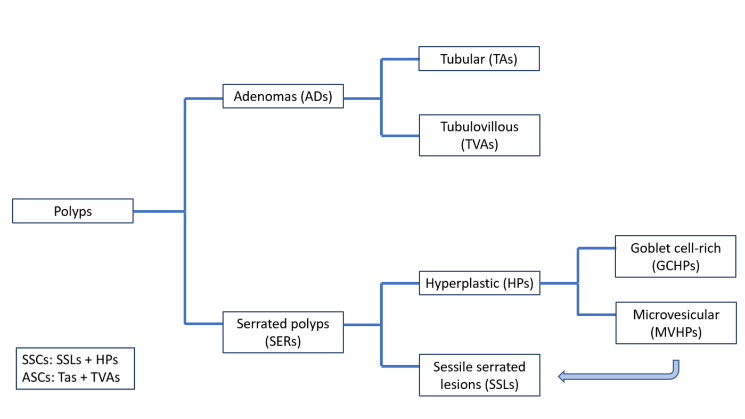
Figure 3 – Subtypes of Colorectal Cancer polyps.
Previous studies found that MSS CRCs show truncating mutations of APC, which result in the activation of the WNT-pathway, and chromosomal instability. It is important to note that WNT is one of the most important pathways of the intestinal epithelium: it is extremely active at the base of the crypts where stem cells are localized, and it takes part in cell renewal and the maintenance of stemness. In addition, MSS CRCs are characterized by gain-of-function mutations in oncogenes (i.e. KRAS) and loss-of-function mutations in tumor suppressor genes (i.e. TP53).
In this study, Chen et al. were able to prove that, during the progression from pre-cancer to MSS CRC, Adenoma Specific Cells (ASCs) maintained an altered expression of WNT and stem genes, while fetal and embryonic gene signatures are acquired only later in MSS CRCs. These results reflect the Vogelstein model, also showing an increase in stemness during tumorigenesis.
On the other hand, the molecular mechanisms that take part in the transition from serrated polyps to MSI-H CRCs are not fully understood. It is known that MSI-H CRCs show epigenetic disruption, with an hypermethylation of MLH1, and a 40-70% prevalence of CpG Island Methylator Phenotype. In contrast to ADs, serrated polyps do not arise from APC mutations, instead, they harbor BRAF mutations. Another characteristic of serrated polyps is a neoantigen-triggered cytotoxic immune infiltration.
In this study, using scRNA-seq analysis, it was possible to affirm that Serrated Specific Cells (SSCs) show a mixed cellular identity, with the expression of genes not usually present in the colon as well as Goblet cell genes (e.g. MUC5AC, MUC2). Furthermore, they observed a downregulation of the negative regulator of cell differentiation and proliferation CDX2, and an unusual expression of the fetal gene MDK.
Performing Multiplex Imaging, using stem cell markers (OLFM4, SOX9, CDX2), the Authors were able to define a mutual exclusivity between the areas stained by the markers and the area stained using MUC5AC. The expression of the MUC5AC gene, which is usually expressed in the stomach, made it possible to hypothesize that MSI-H CRCs tumoral cells may undergo dedifferentiation, implicating a metaplastic tumorigenic process.
Performing computational analysis, to further investigate the phylogenetic origin of SSCs, the Authors were able to define that this cell subtype is more genetically related to the absorptive lineage cells (ABS). This finding implies that, during the tumorigenic process, MSI-H CRCs tumoral cells arise from developmentally committed cells, once again confirming their metaplastic origin.
To investigate the presence of a distinct immune microenvironment in MSI-H CRCs, the Authors performed an in vivo experiment on genetically engineered mice, followed by Multiplex Immunofluorescence. Two distal colon adenomatous tumor models were created: one deriving from stem cells and the other deriving from non-stem cells. From this experiment it was possible to appreciate the presence of an infiltration of CD3+/CD8+ and CD3+ immune cells in the non-stem tumor specimens.
In conclusion, the results imply the existence of two distinct tumorigenic processes. The first one leads to an acquisition of stemness, resulting in the transition from ADs to MSS CRCs. The second tumorigenic process, through dedifferentiation and the acquisition of fetal and metaplastic gene signatures, results in the transition from SERs to MSI-H CRCs. In addition, the immune infiltration of cytotoxic cells can be due to the interaction of luminal stressors with SERs. This interaction causes the activation of DNA repair mechanisms reliant on cellular plasticity, further confirming the hypothesis of a metaplastic tumorigenic model.
The results highlight the presence of specific MSI-H CRCs biomarkers that could have an important role in early diagnosis of suspect SERs. Further studies are needed to determine whether the acquisition of stemness in MSI-H CRCs impacts the likelihood of an immunotherapeutic response and whether the role of the microbiota may open novel strategies for pre- and pro- biotic therapies.
The influence of the microbiota on human health has seen an increase in the scientific community’s interest in recent years. Prebiotics are the nutrients necessary for probiotics: the ease of production and delivery make them a suitable option for the maintenance of a healthy intestinal microbiota. It would be interesting to understand whether the application of pre- and pro- biotics can have a prevention role: luminal stressors, that are responsible for the initiation of cellular damage response, can have a microbial nature. A pre- and pro- biotic supplementation may be useful to rebalance the microbiota in subjects who are predisposed to the formation of intestinal lesions.
References
- McGranahan N, Swanton C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell. 2017 Feb 9;168(4):613-628. doi: 10.1016/j.cell.2017.01.018. PMID: 28187284.
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759-67. doi: 10.1016/0092-8674(90)90186-i. PMID: 2188735.
- Chen B, Scurrah CR, McKinley ET, Simmons AJ, Ramirez-Solano MA, Zhu X, Markham NO, Heiser CN, Vega PN, Rolong A, Kim H, Sheng Q, Drewes JL, Zhou Y, Southard-Smith AN, Xu Y, Ro J, Jones AL, Revetta F, Berry LD, Niitsu H, Islam M, Pelka K, Hofree M, Chen JH, Sarkizova S, Ng K, Giannakis M, Boland GM, Aguirre AJ, Anderson AC, Rozenblatt-Rosen O, Regev A, Hacohen N, Kawasaki K, Sato T, Goettel JA, Grady WM, Zheng W, Washington MK, Cai Q, Sears CL, Goldenring JR, Franklin JL, Su T, Huh WJ, Vandekar S, Roland JT, Liu Q, Coffey RJ, Shrubsole MJ, Lau KS. Differential pre-malignant programs and microenvironment chart distinct paths to malignancy in human colorectal polyps. Cell. 2021 Dec 22;184(26):6262-6280.e26. doi: 10.1016/j.cell.2021.11.031. Epub 2021 Dec 14. PMID: 34910928; PMCID: PMC8941949.