Genetic engineering of human fetal and adult liver organoids by CRISPR-Cas9 technology
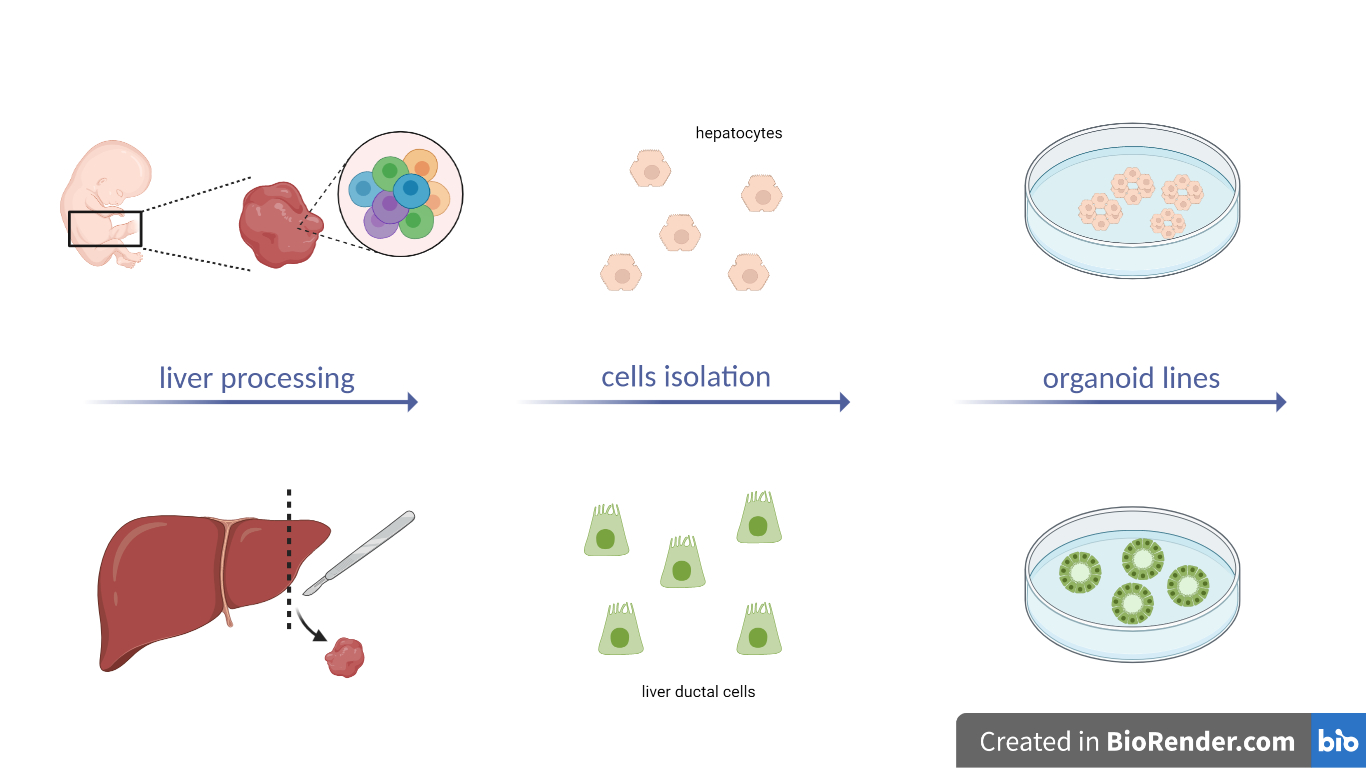
Fig. 1 – Schematic overview of this protocol (Created with BioRender.com)
Abstract
The liver is composed of two types of epithelial cells: hepatocytes and liver ductal cells. However, until recently primary human hepatocytes (PHHs) were challenging to expand in vitro due to the difficulty of introducing genetic material to PHHs cultures while preserving their unique functions and characteristics, restricting researchers from studying these processes in greater detail. In this protocol, the authors provide full details on how to overcome this struggle. Using a CRISPR-Cas9 and homology-independent organoid transgenesis (CRISPR-HOT) approach, efficient gene knock-in can be achieved in organoid lines. The protocol for establishing human fetal hepatocyte organoid cultures takes about 1-2 months, whereas the genome engineering of human liver ductal organoids and human fetal hepatocyte organoids takes 2-3 months. These gene knock-in and knock-out approaches, and their multiplexing, will be useful for a variety of applications such as modelling diseases, studying gene functions and biological processes.
Introduction
In November 2020, a group of researchers from the Hubrecht Institute (the Netherlands) published on “Nature Protocols” a protocol regarding the establishment of human fetal hepatocyte organoids and the application of CRISPR-Cas9-based gene knock-in and knock-out techniques in organoid cultures of human liver [1].
It is well known that human liver organoids could be an excellent model to study hepatic diseases, liver development, to do drug screening, and are suitable for gene editing applications.
Liver organoids can be derived from various cells of different origin by regulating signalling pathways during isolation and seeding of cells into cultures. They can be generated from pluripotent stem cells, either induced pluripotent stem cells (iPSCs) or embryonic stem cells (ESCs), usually by a three-stage differentiation process that recapitulates the signalling programmes active during development [2]. Moreover, liver organoids can be formed from tissue-resident cells isolated from biopsies of adult or embryonic tissues, which is the main approach used in this work.
In this protocol, the authors provide detailed procedures for the long-term expansion of human fetal hepatocytes organoids and genome engineering strategies for both human liver ductal organoids and human fetal hepatocytes organoids.
Discussion
The protocol can be broken down into three different procedures.
Establishment of human liver organoid lines
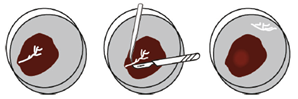
As the first step, researchers point out how to dissect human fetal liver tissue and isolate human fetal hepatocytes. It is mandatory to use fresh material and process it as soon as possible after receiving the liver. If white tree-like structures appear in the liver parenchyma (Fig. 2), they should be removed to prevent the contamination of ductal organoid outgrowth. Next, a combination of collagenase type IV digestion and manual mincing is employed to isolate the hepatocytes. It is crucial to determine the appropriate enzymatic activity and the correct timing of digestion to obtain high-quality hepatocytes that will lead to successful line establishment. Additionally, enrichment through low-speed centrifugation is necessary, followed by mixing the cells with Basement Membrane Extract (BME) culture medium and placing them into small droplets. Overall, careful attention to culture conditions and the addition of specific growth factors (such as FGF-7, FGF-10 or TGFα) can promote the successful development of stable organoid lines [3].
R-spondin-1 conditioned medium (RSPO1-CM) is commonly used for culturing organoids, as it contains proteins that activate the Wnt/β-catenin pathway, which is important for cell growth and differentiation. In addition to RSPO1-CM, the inclusion of CHIR99021, an inhibitor of GSK3B, allowed the researchers to achieve even stronger activation of the Wnt pathway within the organoids.
Organoids will appear after 3-4 days presenting a dense morphology, typically spherical or more grape-like structures. Cystic ductal-like organoid outgrowth (Fig. 3) must be removed from the culture, as their presence impairs the establishment of a pure hepatocytes organoid culture.
From immunofluorescence and RNA sequencing analysis of the established organoid lines, the researchers found abundant expressions of typical hepatocytes markers, such as ALB and SERPINA1. These human fetal hepatocytes organoids also show superior engraftment in mouse liver in vivo.

Fig. 3 – Cystic ductal-like organoid outgrowth (modified from: Hendriks, D., Artegiani, B., Hu, H. et al. Establishment of human fetal hepatocyte organoids and CRISPR–Cas9-based gene knock-in and knock-out in organoid cultures from human liver. Nat Protoc 16, 182–217 (2021) https://doi.org/10.1038/s41596-020-00411-2)
Genome engineering strategies
The second part of the protocol regards genome engineering. Specifically, gene knock-in is carried out through the CRISPR-HOT technique, which uses non-homologous end joining (NHEJ) and requires a targeting plasmid with exogenous DNA linearized by SpCas9 and an sgRNA sequence. Endogenous C-terminus tagging is used to identify the target gene [4]. For gene knock-out, frameshifting indels are introduced using CRISPRCas9-induced double strand breaks (DSB) and NHEJ-mediated imprecise repair. The protocol included identifying and targeting the specific exons with designed sgRNAs cloned into a plasmid.
Two different transfection approaches are described. In the first one, individual cells from PHH-liver-organoids are treated with an accutase solution, mixed with DNA, electroporated, and plated for organoid formation. The cells are checked for fluorescence signals to confirm successful electroporation and can be selected using Fluorescence Activated Cell Sorting (FACS) analysis or drug resistance. In the second transfection method, culture conditions for expansion of human liver ductal cells in vitro as organoids are established as previously described in another protocol [5], intact liver ductal organoids are grown until a proper size is reached, and DNA is injected into the organoids. After successful electroporation, clonal liver ductal organoid lines can be selected using either fluorescence or drug selection. The organoids can be regrown and appear as regular round cysts (Fig. 4). Genotyping of genome-engineered organoids is then performed by PCR.

Fig. 4 – First line: genome engineering of human fetal hepatocyte organoids. Second line: genome engineering of human liver ductal organoids (Created with BioRender.com)
Multiple genomic targeting
The last procedure concerns further genome engineering strategies to obtain multiple gene editing.
For the generation of double or multiple knock-in lines with CRISPR-HOT, the tagging of endogenous genes must be performed sequentially. This is mandatory, because insertion of the desired tag at the right locus is uncontrollable and the probability of simultaneous insertion of each tag correctly at each different locus is very low.
For the generation of double or multiple gene knock-out lines, two options can be considered. First, multiple sgRNAs can be co-electroporated together in a single experiment to generate multi-gene knock-out organoids in a single experiment; alternatively, if the mutations are to be in sequential order, another or multiple rounds of gene knock-out can be performed on an established knock-out organoid line.
Conclusions
Liver organoids are ideal seeds for future liver transplantation in regenerative medicine. They are promising models for our understanding of organogenesis and liver regeneration. Moreover, organoids derived from hepatopathy or liver cancers can be used for disease modelling and drug screening in vitro, fostering the development of personalised treatments.
Despite the promising features of organoids, their broad utility is tempered by a variety of limitations yet to be overcome. These include, for instance, the limited scalability of the process, which has to be reproduced with the necessity of having a large quantity of cells; or the complexity and the elevation of the costs for a technique which is still in the early stages of development.
Nevertheless, further research and technological improvements are being developed to overcome current limitations and efficiently integrate liver organoids into clinical applications.
References
- Hendriks, D., Artegiani, B., Hu, H. et al. Establishment of human fetal hepatocyte organoids and CRISPR–Cas9-based gene knock-in and knock-out in organoid cultures from human liver. Nat Protoc 16, 182–217 (2021) https://doi.org/10.1038/s4.1596-020-00411-2
- Prior N., Inacio P., Huch M. Liver organoids: from basic research to therapeutic applications. Gut. 2019 Dec;68(12):2228-2237. doi: 10.1136/gutjnl-2019-319256. Epub 2019 Jul 12. PMID: 31300517; PMCID: PMC6872443
- Hu H., Gehart H., Artegiani B., LÖpez-Iglesias C., et al. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids, Cell, Volume 175, Issue 6, 2018, Pages 1591-1606.e19, ISSN 0092-8674, https://doi.org/10.1016/j.cell.2018.11.013
- Artegiani, B., Hendriks, D., Beumer, J. et al. Fast and efficient generation of knock-in human organoids using homology-independent CRISPR–Cas9 precision genome editing. Nat Cell Biol 22, 321–331 (2020). https://doi.org/10.1038/s41556-020-0472-5
- Broutier L, Andersson-Rolf A, Hindley CJ, Boj SF, Clevers H, Koo BK, Huch M. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat Protoc. 2016 Sep;11(9):1724-43. doi: 10.1038/nprot.2016.097. Epub 2016 Aug 25. PMID: 27560176