New Culture Condition for Human Intestinal Organoids allowing Secretory Cells appearance and Self-Renewal Capacity maintenance
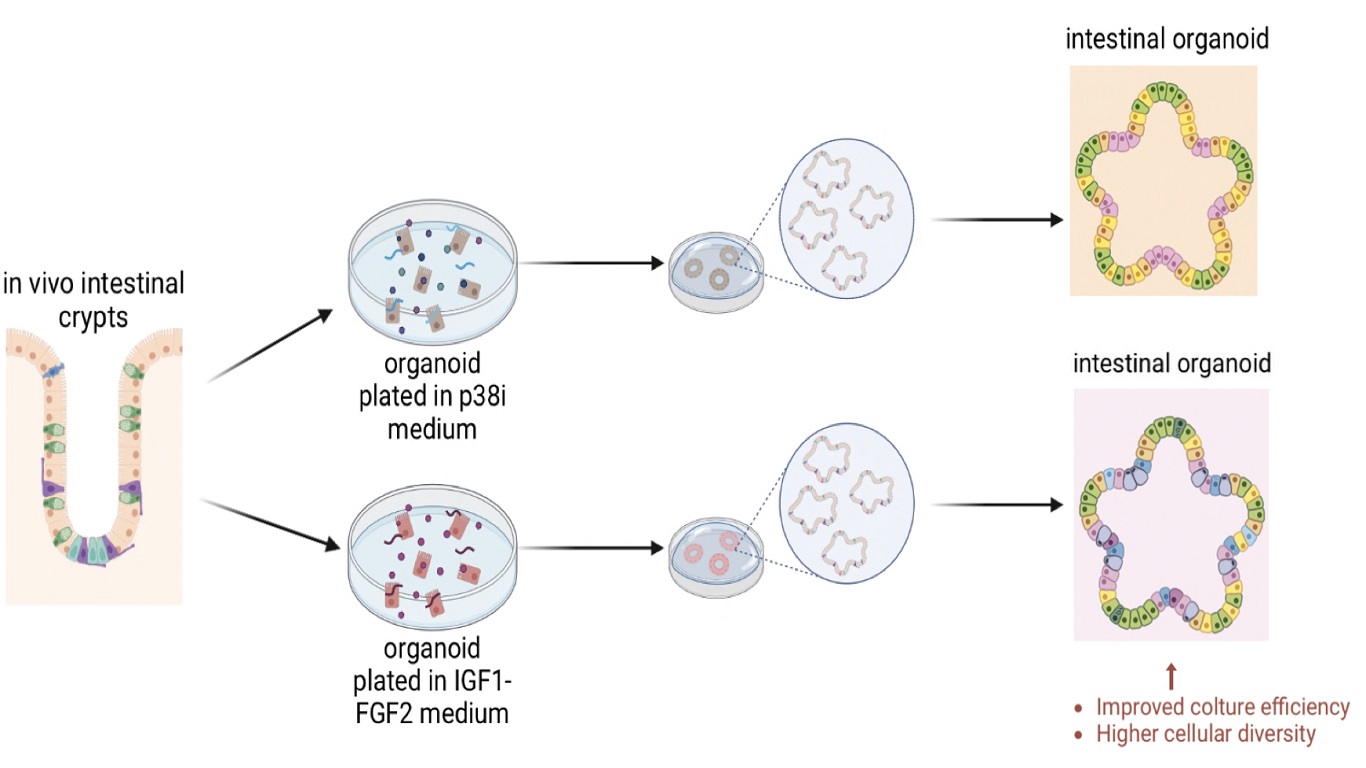
Figure 1. Schematic comparison between IF-cultured organoids and those cultured with the p38i-medium, highlighting the IF-medium advantages. [Image created with Biorender.com © 2023 all rights reserved]
Abstract
The presence of p38-inhibitor in the growth medium for the intestinal organoids impedes the differentiation toward secretory cells, leading to a reduced similarity of the in vivo tissue. To overcome this limitation Fuji, Matano et al. [1] developed a new culture condition for intestinal organoids where the couple IGF1-FGF2 was able to substitute the p38i. The developed p38i-free medium allowed the appearance of all the secretory cell types present in the human intestinal crypts for a period of over six months in culture, proving the multi-differentiation and self-renewal capacity maintenance.
Discussion
Intestinal organoids culturing
Intestinal organoids are 3D cellular culture reproducing in vitro the intestinal lumen and the surrounding intestinal epithelium present in vivo [2]. This type of organoids is particularly subjected to the trade-off effect that consists in the impossibility to obtain the right degree of differentiation maintaining at the same time their self-renewal capacity. The trade-off effect is due to the intestinal organoids’ rigid dependency on p38i (p38 mitogen activated protein kinase inhibitor), whose administration is necessary for intestinal organoids long term expansion but suppresses the differentiation toward secretory cells [3]. To overcome this limitation, the researchers Fujii and Matano, with the help of their collaborators [1], investigated the possibility of a p38i-free medium that could allow both long-term culture and secretory cells appearance. Precisely, p38i blocks the degradation of the epidermal growth factor receptor (EGFR) after the EGF binding by inhibiting the activation of the Cbl ubiquitin ligase. This led the researchers to explore other niche ligands alternative to EGF to overcome the p38i dependency.
Screening of novel growth factors
Fujii and Matano started by screening for the Receptor Tyrosine Kinase (RTK) ligands expressed by the in vivo-isolated human intestinal fibroblast and for the RTKs expressed in human colonic organoids (hCOs). The researchers considered only the ligand that had a pairwise upregulation with the corresponding receptor. This because a ligand that does not have its receptor expressed in intestinal organoids with high probability would not have any effect on their growth and development.
They made an exception in the case of Insulin Growth Factor 1 (IGF-1) since, even if it is not highly expressed by the intestinal fibroblasts, it is present at high levels in the blood system [4].
The selected ligands were singularly tested and showed to be not sufficient for the long-term substitution of p38 inhibitor. In consequence, the researchers proceeded checking if some RTK ligands combinations could bypass the p38i requirement. Precisely, they used a high-throughput screening of every possible combination of two ligands measuring their effect on hCOs growth. IGF-1 coupled with EGF, HB-EGF, FGF-1, FGF-2, FGF-7, or HGF gave the best results in term of organoid area increase.
The identified ligand couples were also shown to promote the growth of other types of intestinal organoids, even those that had been difficult to culture using the p38i-medium, such as certain kind of colorectal tumour organoids.
Among the possible ligand couples, the authors selected for the further experiments, the IGF-1 and FGF-2 combination (IF) for the recombinant ligands’ affordability. The organoid plating efficiency was seen to be further improved when IGF-1 and FGF-2 were combined with EGF (EIF).
Testing for self-renewal capacity and secretory cells in IF cultured organoid
The researchers could validate the self-renewal capacity of IF and EIF cultured organoid by the identification of LGR5+ stem cells on the bottom of the organoid’s crypts. Precisely, the stem cells were detected by a LGR5-reporter knock-in line previously developed using CRISPR-Cas9 technology [5].
The researchers next determined whether the p38i-free medium allows secretory cell type differentiation. Immunoassay using antibody anti-lysozyme for Paneth cells, anti-Chromogranin for enteroendocrine cells and anti-Mucin2 for goblet cells proved the presence of secretory cells in different types of intestinal organoids cultured with IF or EIF. They also noted that enteroendocrine cells were reduced in the EIF medium respect to the IF.
As last step, the researchers performed a single cell RNA sequencing of the IF- or p38i-cultured organoids and compared them by analysing the expression signature of each cell types present in the fresh isolated human intestinal crypts. The p38i-cultured organoid mostly present 3 cell types that are Stem cells, transit amplifying (TA) cells and early enterocytes. Remarkably, the IF-cultured organoid harboured most of the cell types present in the fresh crypts, with the only exception of the mature enterocytes.
IGF-1 and FGF-2 improve intestinal organoids culturing
In conclusion the IF-condition is well-suited for replacing the p38i-mediums, since it allows the formation of intestinal organoids that better mimic the in vivo tissue complexity.
The IF-cultured organoids must now be further tested to assess their ability in modelling intestinal physiological and pathological conditions. In addiction it is necessary to better investigated if the IF couple can also be applied to non-human intestinal organoid models.
Lastly, is intriguing the possibility to further improve the IF condition for example obtaining organoid models presenting also the mature enterocytes to promote their suitability to pre-clinical applications.
References
- Fujii, M., Matano, M., Toshimitsu, K., Takano, A., Mikami, Y., Nishikori, S., Sugimoto, S., & Sato, T. (2018). Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition. Cell stem cell, 23(6), 787–793.e6. https://doi.org/10.1016/j.stem.2018.11.016
- Wallach, T. E., & Bayrer, J. R. (2017). Intestinal Organoids: New Frontiers in the Study of Intestinal Disease and Physiology. Journal of pediatric gastroenterology and nutrition, 64(2), 180–185. https://doi.org/10.1097/MPG.0000000000001411
- Frey, M. R., Dise, R. S., Edelblum, K. L., & Polk, D. B. (2006). p38 kinase regulates epidermal growth factor receptor downregulation and cellular migration. The EMBO journal, 25(24), 5683–5692. https://doi.org/10.1038/sj.emboj.7601457
- Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004 Jul;4(7):505-18. doi: 10.1038/nrc1387. PMID: 15229476. https://pubmed.ncbi.nlm.nih.gov/15229476/
- Shimokawa, M., Ohta, Y., Nishikori, S., Matano, M., Takano, A., Fujii, M., … & Sato, T. (2017). Visualization and targeting of LGR5+ human colon cancer stem cells. Nature, 545(7653), 187-192. https://www.nature.com/articles/nature22081